THE INFLUENCE OF CONSERVATION TREATMENTS AND ENVIRONMENTAL STORAGE FACTORS ON CORROSION OF COPPER ALLOYS IN THE ANCIENT ATHENIAN AGORAALICE BOCCIA PATERAKIS
4 ANALYSIS OF CONSERVATION COATINGSExamination of the North Slope collection found that the majority of those objects displaying blue corrosion had not been coated. Those objects, characterized by both abundant corrosion and coatings and which were well in the minority, could not be sampled for the coating without removing corrosion as well. In these cases the corrosion masked the coating during analysis, which greatly reduced the number of objects appropriate for sampling. Isolation of the coating by separation from corrosion was not carried out in this project. Information was obtained on the coatings of only two objects on which the blue corrosion was evident under the microscope. As a result of this survey, the question of whether the coatings (as lacquers and consolidants) may have served a protective function on the chemically cleaned objects called for their identification. Conservation coatings on five objects were analyzed by Fourier transform infrared spectroscopy using the same instrumentation and procedures that were employed in the analysis of the corrosion products. 4.1 COATINGS SAMPLED FROM COPPER ALLOY OBJECTSThe objects sampled for coatings and a description of these coatings are presented in table 10. The object from which sample 1 was removed had been chemically or electrochemically cleaned and “soaked in distilled water … then impregnated with A140” (Agora n.d.). The object was virtually free of corrosion except for one spot of blue corrosion. The object from which sample 2 was removed had been chemically or electrochemically cleaned and coated with an unidentified, waxlike material; it was virtually free of corrosion products. The object from which sample 3 was removed was probably not chemically cleaned, as it preserves malachite and cuprite. The coating was crazed and somewhat brittle over much of the object. Those areas that were not crazed adhered well to the surface and were wax-like. The object from which sample 4 was removed had been consolidated with a thick layer of wax. Some fragments, which appeared to have been chemically cleaned and were free of wax, displayed blue on top of dark brown corrosion. The object from which sample 5 was removed had been chemically cleaned and “soaked in distilled water … impregnated with A140” (Agora n.d.). The object was, for the most part, free of corrosion on the surface except for small crystals of sodium copper carbonate acetate in grooves and on the sides of a hole that pierces the object (i.e., those areas protected from contact with a resting surface or handling). 4.2 RESULTS OF COATING
|
 |
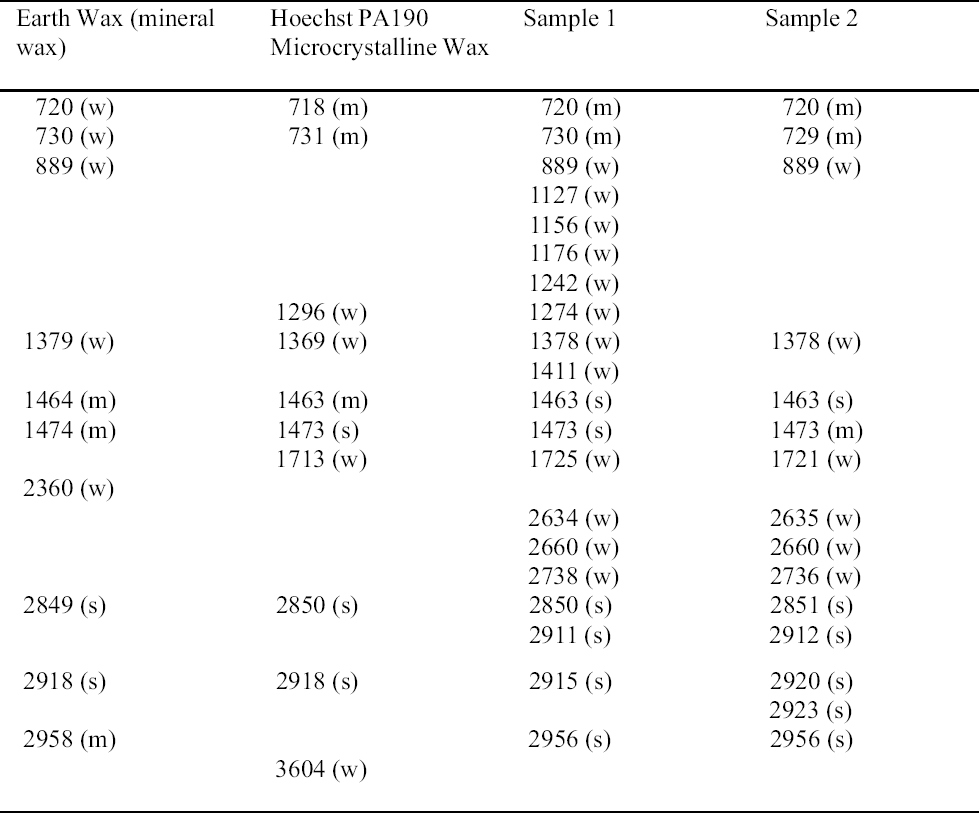
Plenderleith published a wax polish mixture in 1956 that consisted of microcrystalline wax, Cosmolloid 80 Hard (100 g), polyethylene wax, and BASF Wax A (25 g), dissolved in white spirit (Plenderleith 1956). This mixture was prepared by first melting the waxes together and then adding the solvent. By 1971 the mixture was being marketed under the trade name Renaissance Wax (Picreator Enterprises Ltd., London) (Plenderleith and Werner 1971). We may conclude from these results that those individuals treating the objects in the Agora were familiar with the materials used for conservation in the British Museum in the early years, most likely through the publications of Plenderleith and Werner. These publications were also widely used by conservators in America (Drayman-Weisser 1994).
The application of paraffin wax and beeswax (also used in the Agora) to copper alloy objects can cause the formation of organometallic compounds that liberate free fatty acids or dicarboxylic acids. These can combine with copper or lead ions to form metal soaps; copper soaps can promote further corrosion (Paterakis 1996; Scott 2002). Another disadvantage is the irreversibility of wax used for consolidation. As waxes can never be completely removed from a porous object, they interfere with subsequent application of conservation materials. In spite of these drawbacks, it would appear that the hydrocarbon waxes applied to the chemically cleaned metals in the Agora may have protected them from acetic acid attack in storage and the development of acetate corrosion.
4.2.2 CELLULOSE NITRATE
 |
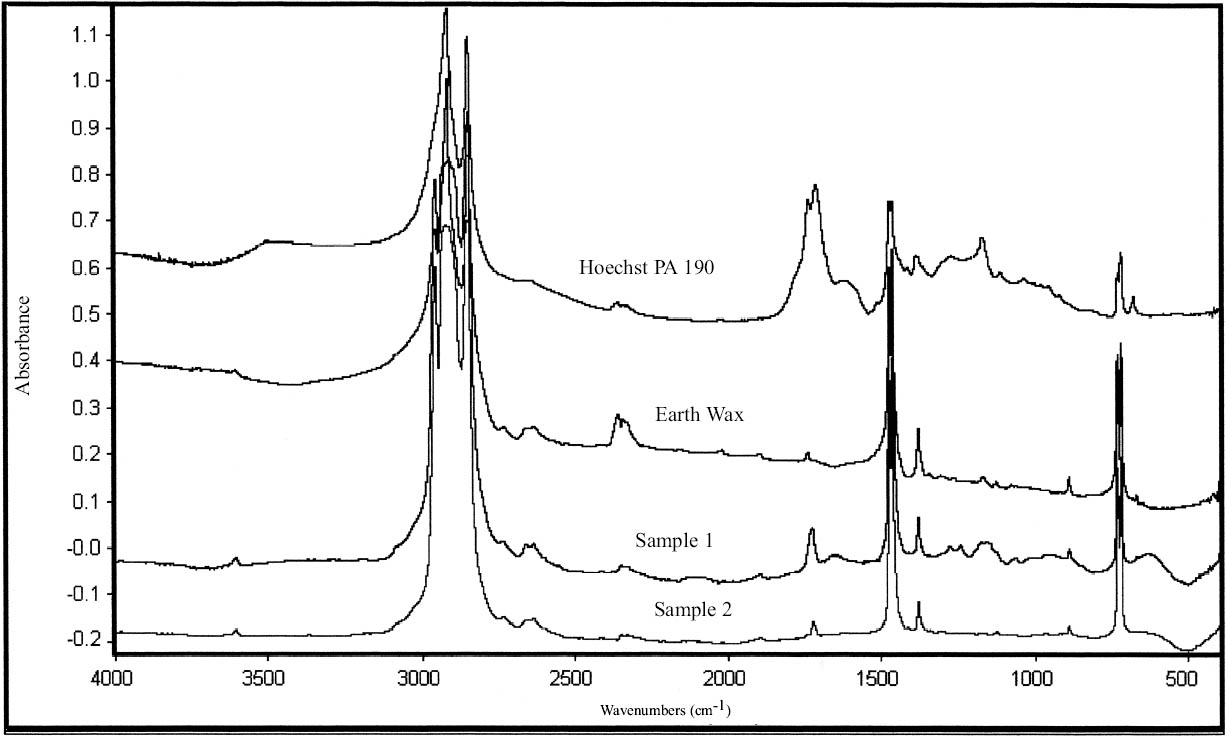
Cellulose nitrate lacquers were made commercially available in 1877 and were first used for conservation in 1899 (Horie 1987; Hatchfield 2002). They have been widely used in the Agora. Cellulose nitrate was identified by FTIR analysis in sample 5 (fig. 9) (table 14). Cellulose nitrate has characteristic asym-metric N=O stretch at 1629 to 1653 cm-1, symmetric stretch at 1285 to 1272 cm-1, N=O stretch from 872 to 841 cm-1, and N=O bending at 761 to 745 and 710 to 689 cm-1 (Thickett 2002). The peak at 1732 cm-1 is a carbonyl stretching due to degradation (Shashoua et al. 1992). Three of the FTIR absorption bands of sample 5—750, 840, and 1277 cm-1—match those of Ercalene, a product that was introduced by Goddards in the 1960s (ICCROM 1963; Scott 2002). Other strong absorption bands
 |
 |