THE INFLUENCE OF CONSERVATION TREATMENTS AND ENVIRONMENTAL STORAGE FACTORS ON CORROSION OF COPPER ALLOYS IN THE ANCIENT ATHENIAN AGORAALICE BOCCIA PATERAKIS
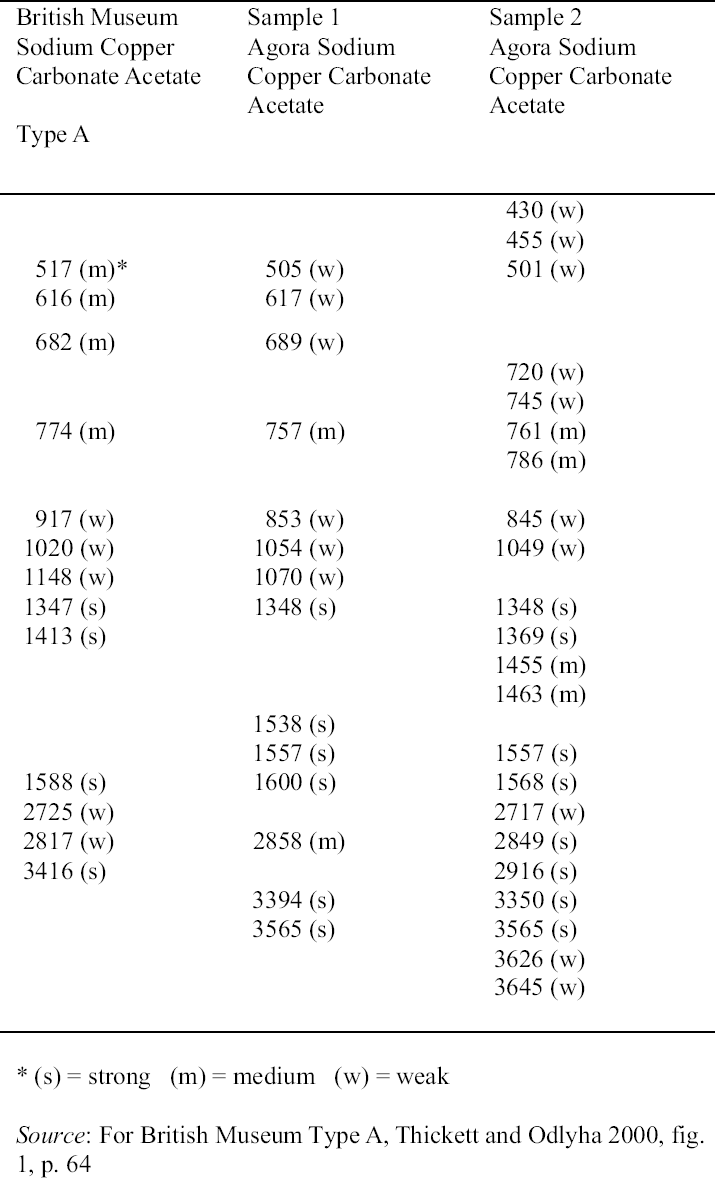
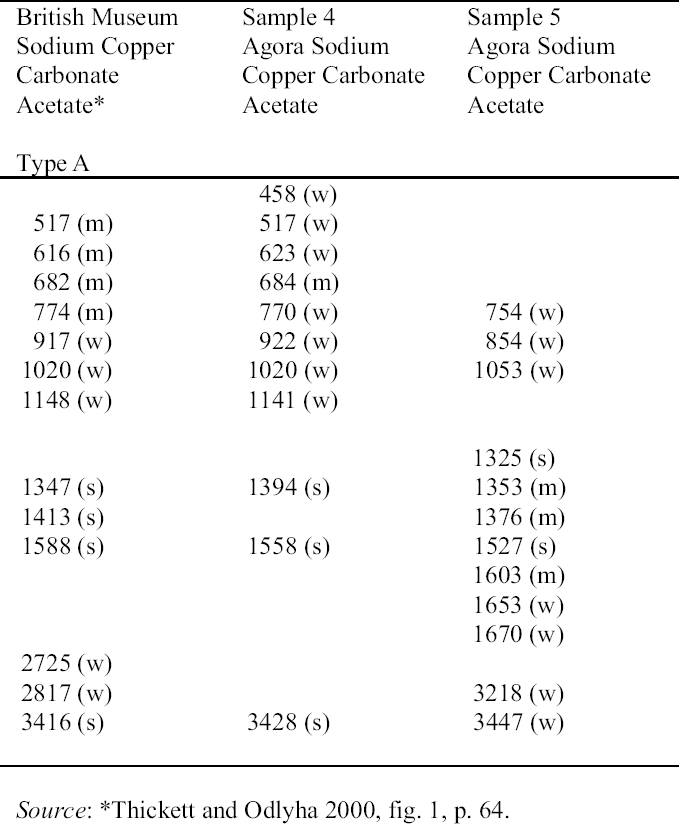
3 FACTORS AFFECTING THE DEVELOPMENT OF THESE CORROSION PRODUCTS3.1 SODIUM COPPER CARBONATE ACETATE AND SODIUM ACETATE TRIHYDRATE3.1.1 Influence of Environmental Storage Conditions: Acetic (Ethanoic) AcidSeveral factors have been identified as contributing to the formation of sodium copper carbonate acetate and sodium acetate trihydrate. Sodium may derive from salt in the burial environment, e.g., sodium chloride, or from incomplete removal of conservation materials used in cleaning or stabilization. The copper derives from the copper alloy,
The objects analyzed from the collections of the Agora and the British Museum had been stored in wooden cupboards since the 1930s. The acetic acid concentration in these wooden cupboards in the British Museum and Agora excavations has been measured. A concentration of 1039 � 20 to 1267 � 20 �gm-3 (400 to 500 ppb at 25�C) acetic acid, found in the Agora excavations, is comparable to the levels found at the British Museum: 1071 to 2880 �gm-3(Paterakis 1998; Thickett et al. 1998). Relative
Acetic acid may be adsorbed onto the surface of an artifact and remain inactive, depending on the relative humidity (Donovan and Stringer 1971). Acetate found in the copper (II) hydroxide corrosion of sample 6 by ion chromatography analysis may have resulted from adsorbed acetic acid vapor. Exposure to higher humidity by transfer of the object to another environment or by an increase in RH in the place of contamination may trigger its activity. In this way, corrosion by acetic acid may occur in locations and conditions not characterized by acetic acid contamination (Donovan and Stringer 1971). The concentration of acetic acid may have been much higher when the storage cases were new (Hatchfield 2002). It should be kept in mind that some objects have had up to 70 years in which to develop the acetate corrosion products, although a relatively short time is required for their formation. In the Burrell Collection the sodium acetate trihydrate developed within a three-year period, whereas the associated blue corrosion products developed within a few weeks (Tennent and Baird 1992). Another source of acetic acid could be artificial patination. Chemically stripped objects were sometimes patinated by dipping them in ammonium acetate or by exposing the object to ammonia or acetic acid vapors (Fink and Eldridge 1925). Farnsworth
3.1.2 Influence of Cleaning and Stabilization Materials on the Formation of the Corrosion Products3.1.2.1 SodiumSodium that has contributed to the formation of sodium copper carbonate acetate and sodium acetate trihydrate in the Agora (Paterakis 1999) may derive from (1) the burial environment, (2) chemical cleaning agents such as Calgon (sodium hexametaphosphate/sodium polyphosphate), zinc and sodium hydroxide, alkaline Rochelle salt (5% sodium hydroxide and 15% sodium potassium tartrate), alkaline glycerol (15% sodium hydroxide and 40% glycerin), (3) the stabilization compounds sodium sesquicarbonate and sodium carbonate, or (4) artificial patination with sodium carbonate or bicarbonate (Tennent and Baird 1992) or with sodium sulfite or sodium thiosulfate (Lucas 1932). Specific weight and volume units for the notations of weight (w), volume (v), and % used throughout this text may be found in appendix I, “Strength of Solutions,” in Plenderleith 1956 (343). The discrepancy between the occurrence of sodium copper carbonate acetate and sodium acetate trihydrate on those objects that were not chemically treated in the British Museum and the absence of these compounds on the analogous objects in the Agora may lie in the burial context of the objects. Those artifacts that exhibited acetate corrosion in the British Museum were from Egypt and thus may have been subjected to higher concentrations of sodium salts from the burial environment. There is little documentation of conservation treatments for individual objects in the Agora prior to 1979, rendering the attribution of chemical cleaning and stabilization agents very difficult. We know from general records that a number of cleaning and stabilization compounds containing sodium have been used over the years. Many of these chemical agents were also used on bronzes in the Ashmolean Museum, Oxford, and the Petrie Museum, London, in earlier years (Jaeschke and Jaeschke 1988; Norman 1988). 3.1.2.2 Alkaline Rochelle SaltA few copper alloy objects in the collection were treated, as recorded in the 1950s, with aqueous solutions of alkaline Rochelle salt: sodium hydroxide and Rochelle salt (sodium potassium tartrate). In 1932 and 1956, respectively, Lucas and Plenderleith recommended
3.1.2.3 Sodium Hexametaphosphate/Sodium Polyphosphate (Calgon)Since the 1930s, copper alloy artifacts at the Agora have often been cleaned with Calgon, in concentrations varying from 10% to 35% in water. It is believed that Calgon was originally sodium hexametaphosphate, the composition of which was later changed to sodium polyphosphate. According to the Calgon website, the main active ingredient today is polycarboxylates (www.calgon.com 2002). Six documented treatments with Calgon in the Agora (objects AB177, B1209, ΞΞ 310–ΞΞ 313) have produced sodium copper carbonate acetate. Farnsworth and Plenderleith mentioned the use of 5–15% sodium hexametaphosphate (Calgon) for removing calcareous deposits as well as malachite from copper alloys (Farnsworth 1940; Farnsworth 1949; Plenderleith 1956). Farnsworth attested to the soaking of several small bronze objects in the Agora for a month or more in a cold 5% solution of Calgon (referred to as sodium metaphosphate in 1940) to remove the calcareous deposit. This Calgon treatment is reported not to have altered the patina. She stated that a hot 10% solution could also be used to accelerate the process (Farnsworth 1940). 3.1.2.4 Sodium Carbonate and Sodium
|
 |
Since 1996, Drayman-Weisser's method of inhibiting bronze disease with sodium carbonate followed by the application of benzotriazole (BTA) has been used in the conservation of Agora copper alloys (Drayman-Weisser 1987). It is used as a 5% (w/v) solution in distilled water. Cuprous oxide (yellow-orange) and sodium copper carbonate (chal-conatronite) (blue-green) are two products of the interaction of sodium carbonate with cuprous chloride in this procedure. These are washed away, and the process is repeated until no more copper oxide is produced (Drayman-Weisser 1987). Another product that can sometimes form on the patina is black cupric oxide (tenorite). It probably forms from the oxidation of the cuprous oxide, produced during the conversion of the cuprous chloride (Drayman-Weisser 1987).
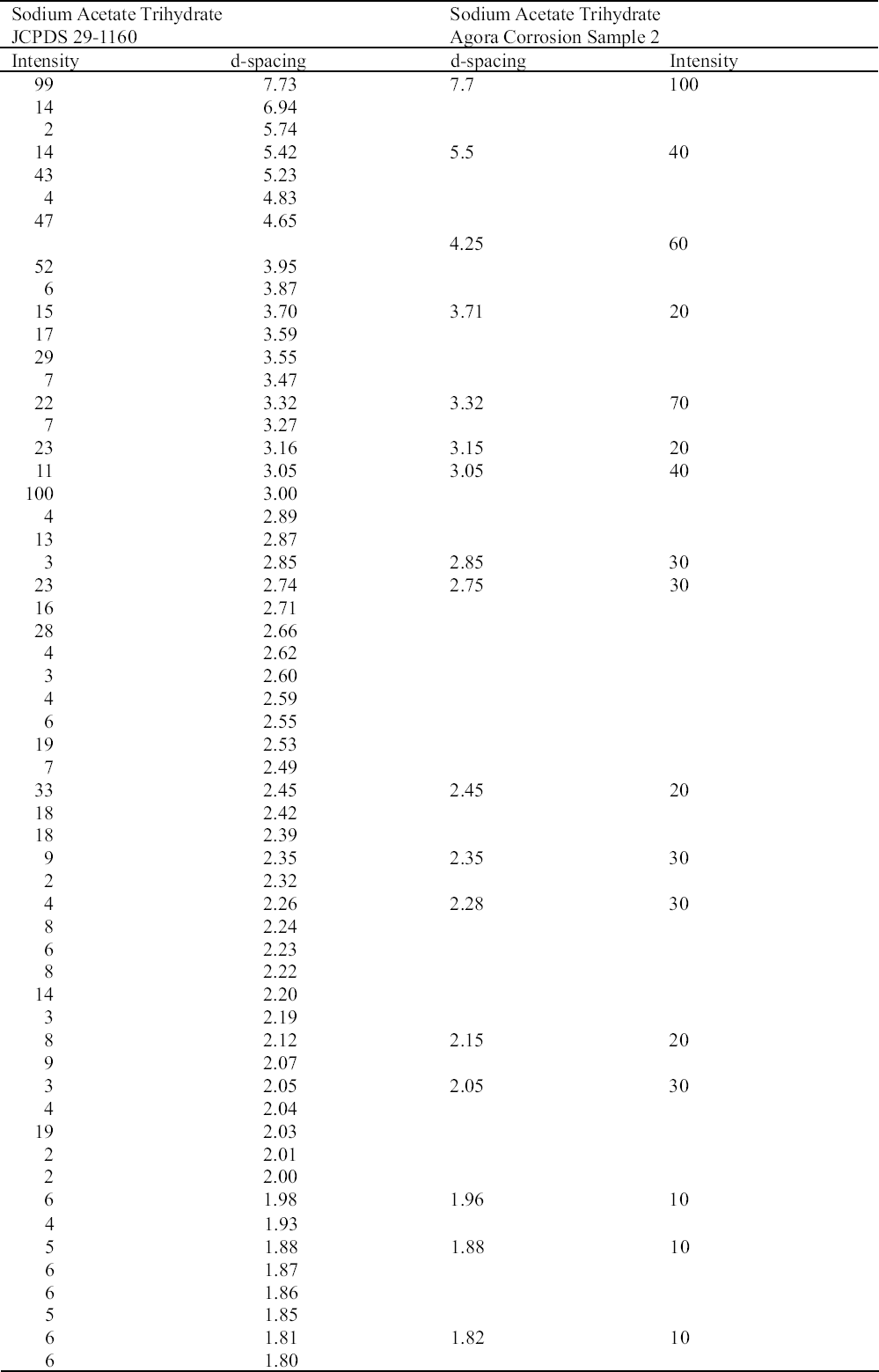
Although there are no specifically documented cases for the use of sodium sesquicarbonate in the Agora, it was very likely to have been used, as it is mentioned in some conservation notes (Witherill 1955). In 1932, Lucas recommended prolonged soaking of copper alloys in a solution of 20 parts (w) sodium sesquicarbonate to 100 parts (v) water. Two years later, Plenderleith (1934) recommended it for cleaning and stabilizing copper alloys. He suggested alternating a 5% solution of sodium sesquicarbonate with a 2% solution of citric acid for cleaning and stabilizing, and a 5–25% solution of sodium sesquicarbonate for removing more tenacious encrustation. He also recommended it for local stabilization of bronze disease. Oddy and Hughes (1970) suggested a 5% solution (w/v) in water. It produces the same products as sodium carbonate: chalconatronite, cuprite, and tenorite (Drayman-Weisser 1987; MacLeod 1987; Pollard et al. 1990; Scott 1997). Chalconatronite may have been present in two samples analyzed by XRD in 1998 (Wheeler 1998). As a hydrated compound, it deliquesces when the RH exceeds the equilibrium relative humidity of the compound and crystallizes when the RH falls below this level. It may absorb acetic acid from off-gassing wooden storage cases to form blue sodium copper carbonate acetate (Paterakis 1999). There have been studies relating the use of sodium sesquicarbonate with the formation of sodium acetate trihydrate (Tennent 1992).
3.1.2.5 Sodium Hydroxide in Electrochemical Cleaning and Electrolysis
Electrochemical reduction by zinc and sodium hydroxide was commonly used at the Agora from 1931 on. The Krefting technique, documented by Rathgen in 1905 and Lucas in 1932, was used for cleaning coins by sandwiching them in between sheets of zinc with a sodium hydroxide electrolyte (Rathgen 1905). This technique has been used extensively in the Agora, sometimes with pellets or granules of zinc, for reducing copper alloy objects, including three coins that display the blue corrosion product (N10763 sample 5, N11172, N11308). It appears that many objects cleaned in this way have a dark brown surface on top of which lies the acetate corrosion. One object contains a trace of zinc observed by qualitative EDS that may have resulted from this electrochemical process. Coins have been cleaned as recently as 1982 using this electrochemical method, followed by benzotriazole (BTA) stabilization and Incralac coating; a small percentage have since developed sodium copper carbonate acetate corrosion.
Electrolytic cleaning was carried out with a 5% solution of sodium hydroxide electrolyte, which may also contribute sodium to the corrosion process. There is one instance of blue corrosion on an object (N10746) that underwent this type of cleaning in 1981, followed by treatment with BTA and coating with Incralac.
Another theory for the formation of sodium copper carbonate acetate, distinct from the theory presented in section 3.1.2.4 regarding chalconatronite, is based on the presumed incomplete reduction of the secondary copper corrosion products, such as malachite, during electrochemical or electrolytic cleaning (Bradley and Thickett 1998; Paterakis 1999). Any remaining malachite could serve as a host for the formation of sodium copper carbonate acetate. It is most likely that two factors regarding chemical cleaning are required for the production of blue sodium copper carbonate acetate: the incomplete reduction
3.2 COPPER (II) HYDROXIDE, SPERTINIITE (TURQUOISE BLUE CORROSION PRODUCT)
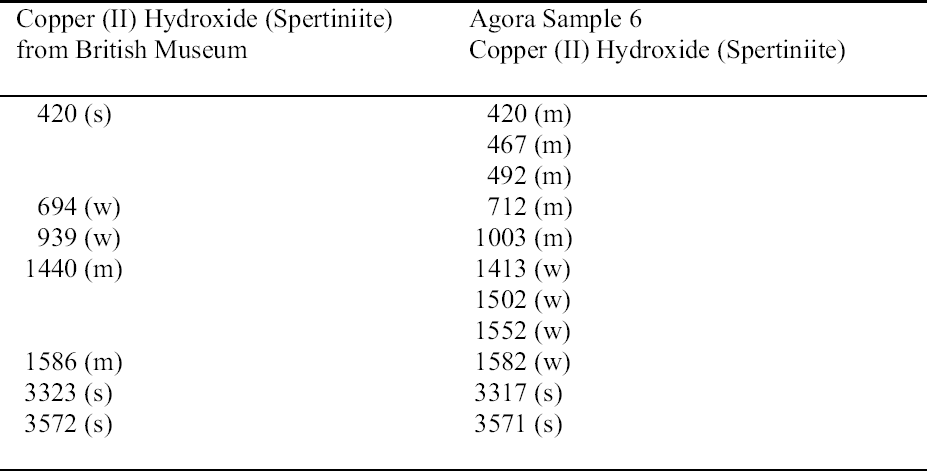
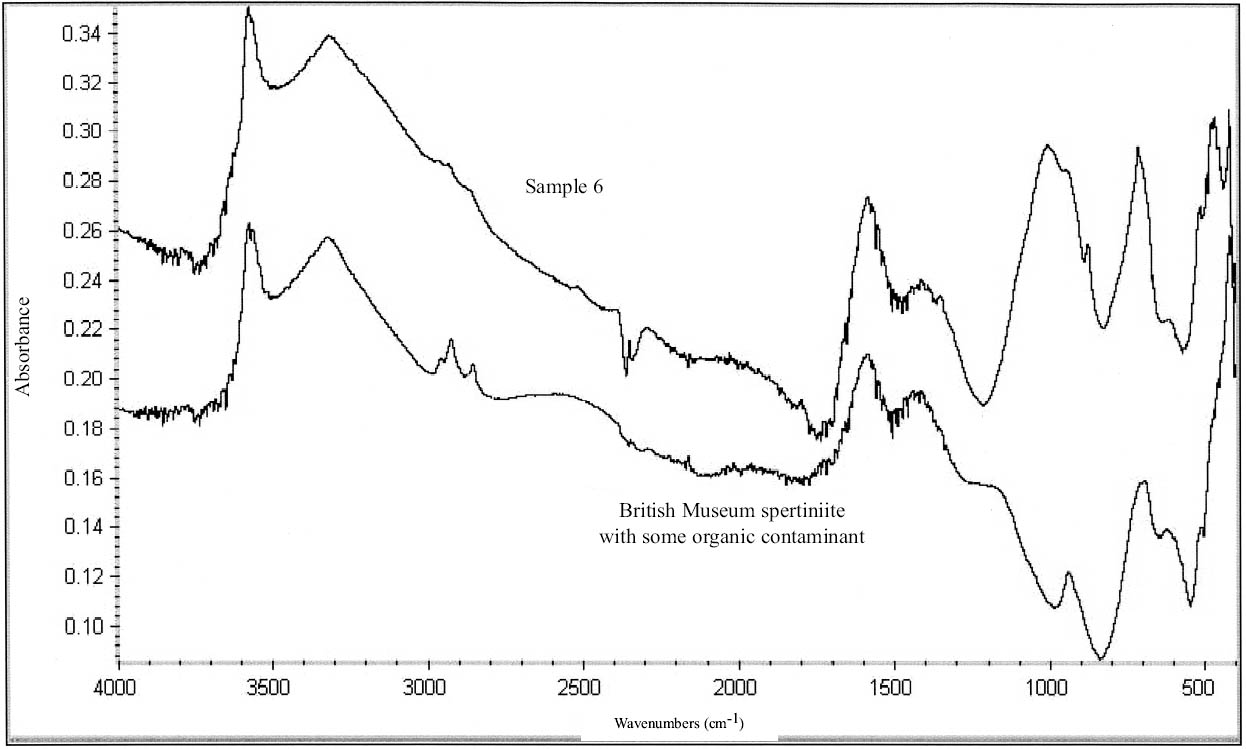
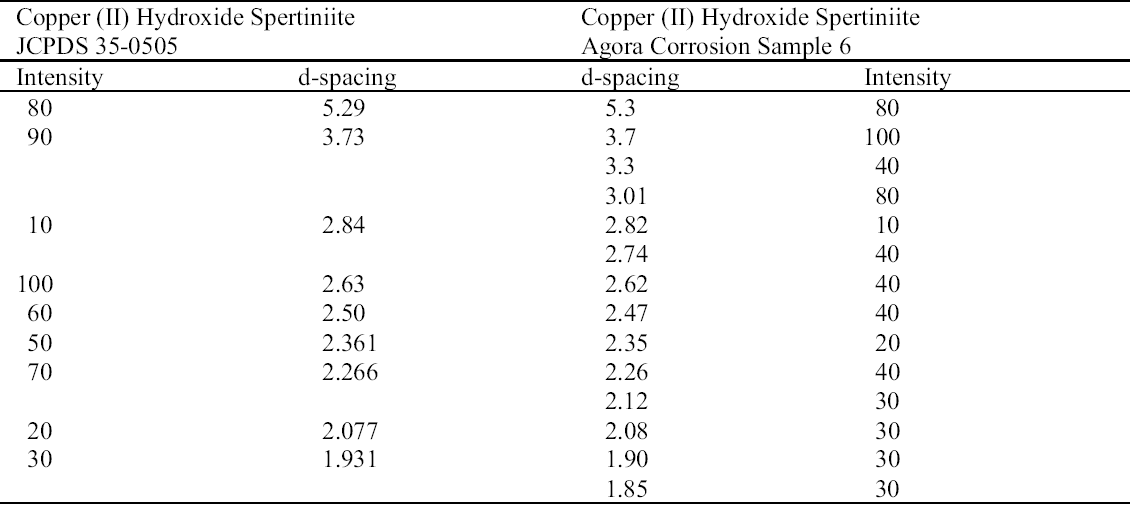
The turquoise blue corrosion product (sample 6) was identified as copper (II) hydroxide, spertiniite (Cu[OH]2) (see fig. 3, page 259). It generally forms on top of the corrosion crust, which is very brittle and readily breaks off, exposing a smooth cuprite surface below. This compound is described as duckegg blue, unstable in the presence of carbon dioxide and water, and poorly crystalline (Scott 1997). In the presence of carbon dioxide it has a tendency to change into copper hydroxy carbonates or, in the presence of chloride, into copper hydroxy chlorides (atacamite, paratacamite) (MacLeod 1999). Only one other instance of copper hydroxide on a copper alloy object has been published, and that was from a marine context (MacLeod 1991). The same compound has been identified by XRD and FTIR on an Asiatic bull in the British Museum (Thickett 1999). If there is a significant amount of moisture present, e.g., in the pores of hygroscopic corrosion products on corroded bronze, the dehydration process will be minimized, thereby stabilizing the copper hydroxide. A high ambient RH may contribute to the moisture present in the corrosion products. In addition to the presence of significant amounts of water, two other factors may support its stability: (1) the local pH and (2) the presence of a mixture of tin (II) and tin (IV) corrosion products on the surface and the presence of degraded metal underneath (MacLeod 1999). MacLeod has found many hydroxy species associated with tin hydrolysis (MacLeod 1999). An additional factor that may stabilize the copper hydroxide is the adsorption of SO2 (MacLeod 1999). The reported insolubility of spertiniite in water was confirmed in the Agora (Weast and Astle 1979).
The use of sodium sesquicarbonate on the object from which sample 6 was taken cannot be ruled out, although sodium was not detected in the analysis. It is possible that copper (II) chlorides and copper hydroxy chlorides, formed from the oxidation of copper (I) chlorides in the corrosion matrix, could be hydrolyzed by sodium sesquicarbonate (depending on the hydroxide concentration in solution), forming copper (II) hydroxide (MacLeod 1999; Paterakis 1999). The fragility of the corrosion crust and the tendency of this crust to separate from a smooth underlying surface may be the result of chemical treatment.
3.3 CASSITERITE AND CUPRITE (DARK BROWN CORROSION)
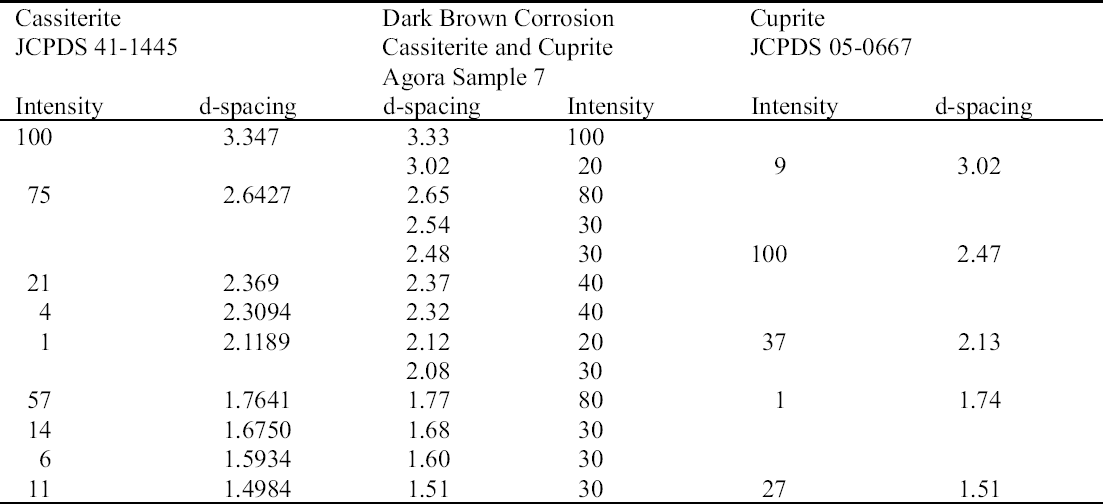
Cassiterite (SnO2) and cuprite (Cu2O) are the major components of the dark brown corrosion layer (sample 7) (see fig. 2, page 258) that can result from various reactions from cleaning as follows. The primary tin and copper corrosion products present in the original corrosion front would have been exposed by the removal of the secondary corrosion products on the surface during cleaning. The primary oxidation product of tin, Sn2+, may be subjected to hydrolysis and precipitated, or the tin (II) may be further oxidized to tin (IV), which can then undergo hydrolysis. Chemical cleaning of tin and copper corrosion products and the removal of copper (I) chloride complexes (e.g., with sodium sesquicarbonate) result in the hydrolysis of soluble tin (IV) and copper (I) materials that yield cassiterite and cuprite. Treatment with a 10% solution of sodium sesquicar-bonate can cause copper to redeposit as cuprite on the surface of the object, which has been made alkaline by the treatment (Scott 2002). In electrochemical or electrolytic reduction, any nonreduced secondary copper corrosion products, such as malachite, form cuprite (Cu2O) and tenorite (CuO), which appear as a black, powdery product (Scott 1997). The tin corrosion products, which generally are not reduced to tin metal, hydrolyze to produce
While the dark brown and blue corrosion products often occur together, they seem to form independently from one another. The only common factor in their formation appears to be sodium, for example from sodium sesquicarbonate, which could have contributed to the formation of cuprite from copper (I) chloride in the dark brown corrosion (Scott 1997) and of sodium copper carbonate in the blue corrosion.
3.4 THE ROLE OF RELATIVE HUMIDITY AND CONSIDERATIONS FOR THE REMOVAL OF CORROSION PRODUCTS
The relative humidity in the Agora storeroom was monitored over a one-year period and was found to reach a minimum of 40% in the summer and a maximum of 82% in the winter. Daily fluctuations ranged from �2% RH in the summer to 17.5% RH in the winter (Paterakis 1990). The buffering capacity of the wooden cupboards should not be discounted in the consideration of RH inside the cases. The sodium copper carbonate acetate in the British Museum was found to deliquesce at a relative humidity of approximately 65%, indicating its equilibrium relative humidity (eqRH); the eqRH of sodium acetate trihydrate was found to be 75% (Thickett 1998).
As relative humidity has been shown to play an important role in the corrosive activity of volatile acetic acid on metal, considerations in the removal of the acetate corrosion products should take into account the equilibrium relative humidity of the compounds. If the ambient RH is less than the eqRH of the acetate compounds, the corrosion will be in crystalline form, but if the RH meets or exceeds the eqRH, the corrosion crystals will deli-quesce and the salt solution will wick into the pores of the artifact (Thickett 1998; Paterakis 1999). This result is to be avoided. In the Agora the equilibrium relative humidity of the acetate compounds is exceeded in the storeroom in the winter months. Long-term stability of the objects as well as their legibility and aesthetic appearance should be considered in the removal of these corrosion products. Dry (mechanical) removal of the crystalline acetate compounds is preferable to wet removal to prevent spreading in the pores of the object. Alternatively, much of the crystalline sodium copper carbonate acetate can be removed with cotton swabs moistened with ethanol, and the crystalline sodium acetate trihydrate can be removed with water-moistened cotton swabs or poultices. There may be no need to remove the dark brown corrosion, as it often follows the configuration of the surface and is normally not disfiguring. The sodium acetate trihydrate and sodium copper carbonate acetate in the Agora are not necessarily disfiguring but do detract from the legi-bility and aesthetic appearance of the objects. Their removal is recommended if the afflicted objects cannot be stored in a stable relative humidity that is low enough to prevent their deliquescence (i.e., less than the equilibrium relative humidity of these acetate compounds). All metal objects in the Agora are currently being relocated to new powdercoated baked-enamel steel cases in a recently constructed dry storage room. Plastic storage containers maintained with low RH by conditioned silica gel should be used as the primary container for copper alloy artifacts if wooden storage facilities cannot be replaced and if the ambient relative humidity cannot be controlled. The copper (II) hydroxide may be removed mechanically, as it is not water-soluble.
3.5 INFLUENCE OF CONSERVATION COATINGS ON THE FORMATION OF THE CORROSION PRODUCTS
Over the years, many materials in the Agora have been applied to copper alloy artifacts, although there is little documentation predating 1979. A small percentage of the North Slope copper alloys have been coated, however, and a smaller percentage of these display sodium copper carbonate acetate corrosion. Lucas stated that it is best not to use preservative coatings (oils, waxes) on copper alloy objects, but that
Acetate polymer coatings are known to have been used in the Agora, and their effects on the formation of acetate corrosion products have been questioned (Paterakis 1998). Polyvinyl acetates were mentioned by Gettens for use in the conservation of artifacts as early as 1935 (Gettens 1935). According to Gettens, in 1935 the vinyl resins were a rather new material with only a 10-year history of use as film-forming substances. Polyvinyl acetates were also used on copper alloys in the Petrie Museum, London, in the 1950s and 1960s (Jaeschke and Jaeschke 1988).
One coating material in particular, Unichrome Lacquer A140, manufactured by the Metal & Thermit Corporation of New York, was of particular interest, as it is one of the few coating materials recorded in early treatments in the Agora that were identified by manufacturer and product name. An analytical project was carried out to identify the coatings used and to determine the effects of these coatings on the corrosion products, in particular on acetate corrosion.