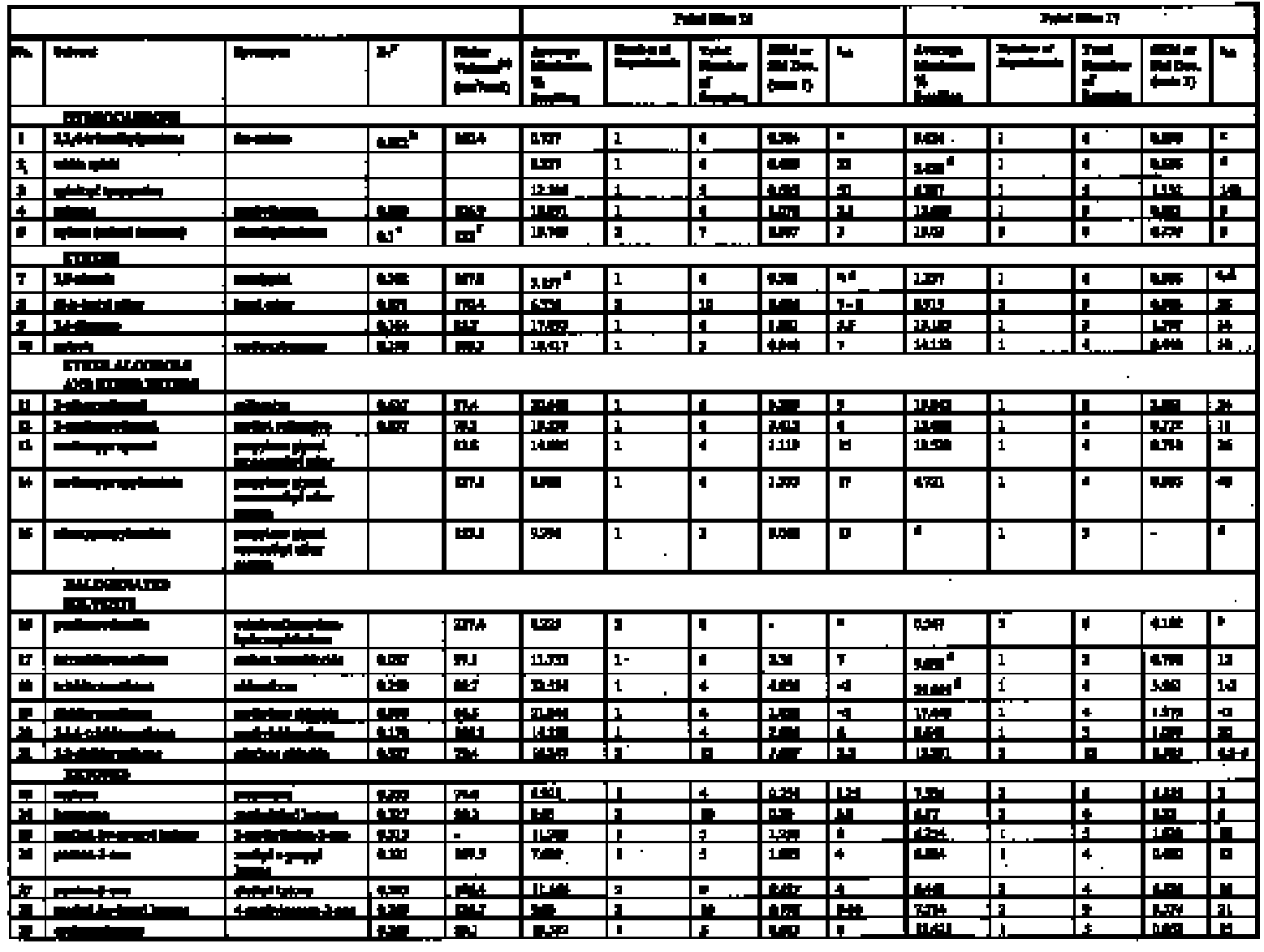
THE SWELLING OF ARTISTS' PAINTS IN ORGANIC SOLVENTS. PART 2, COMPARATIVE SWELLING POWERS OF SELECTED ORGANIC SOLVENTS AND SOLVENT MIXTURESALAN PHENIX
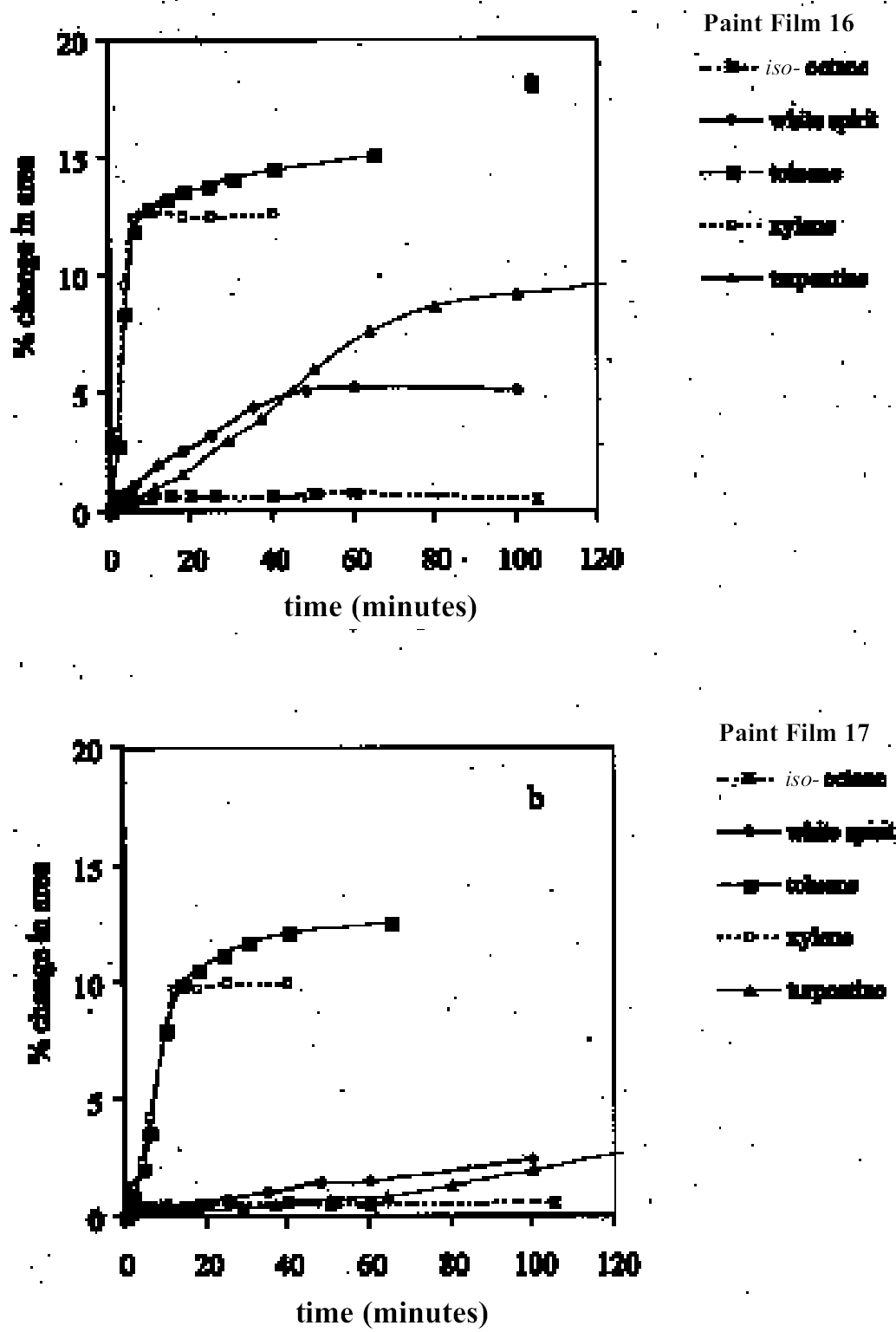
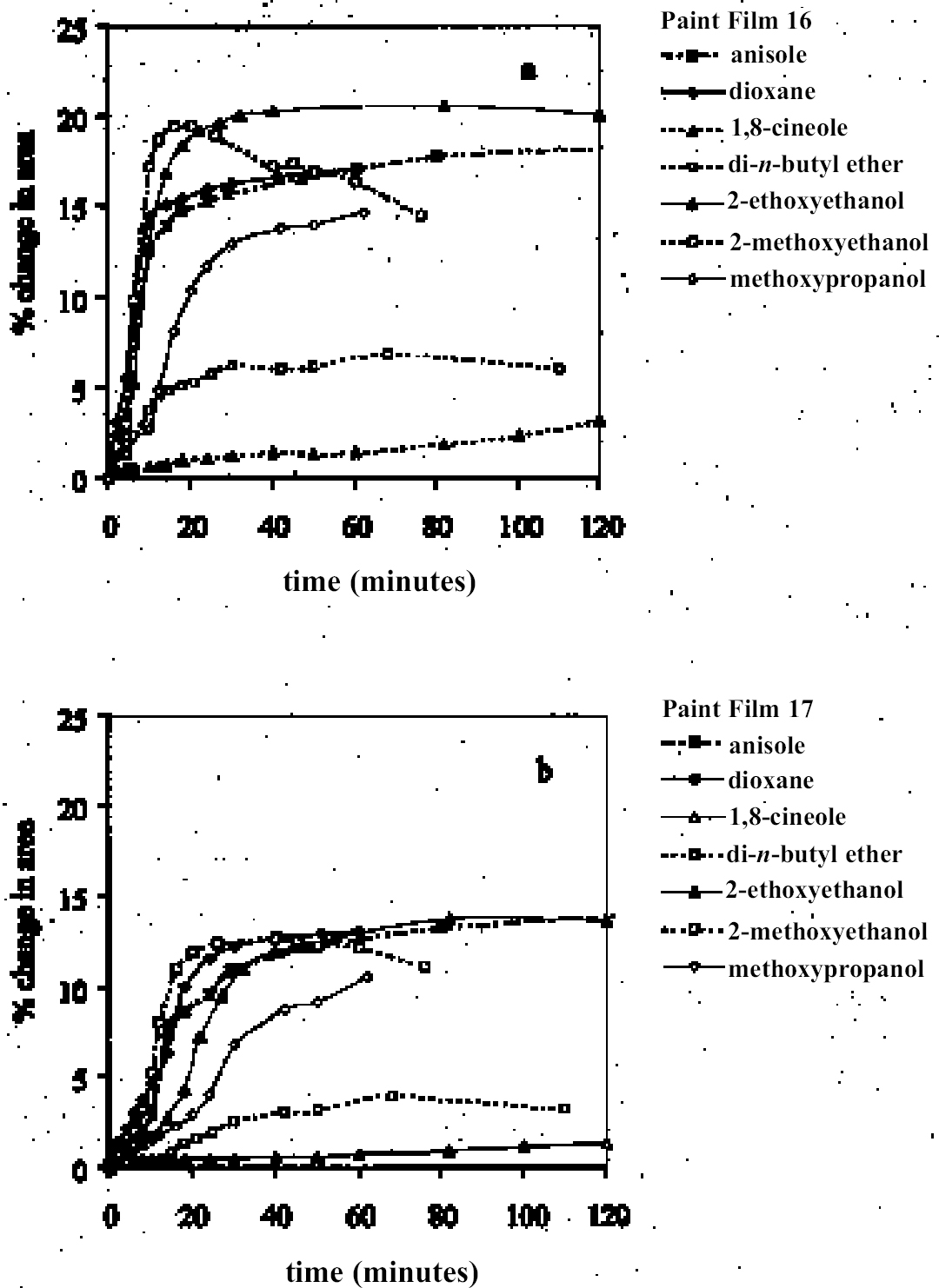
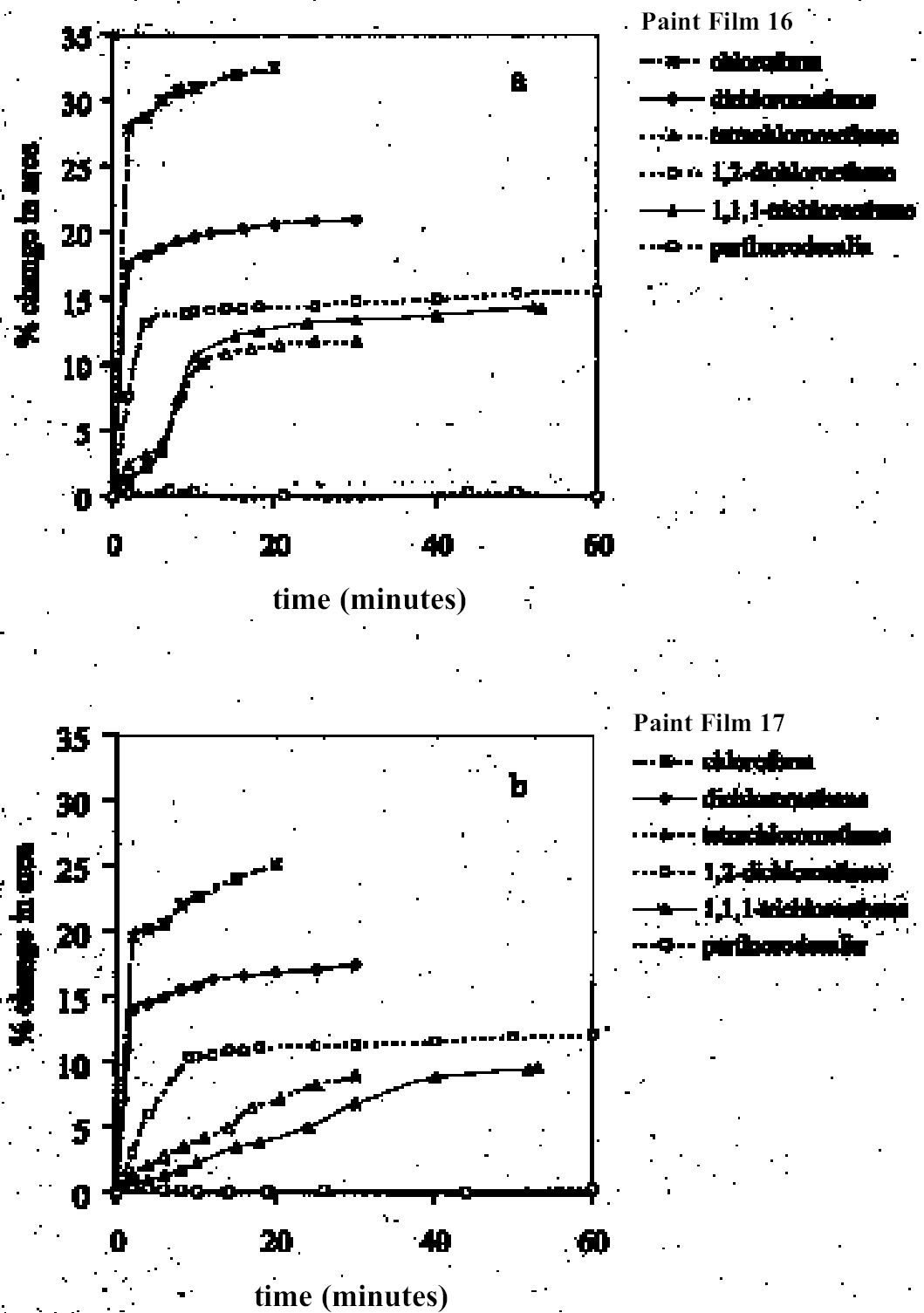
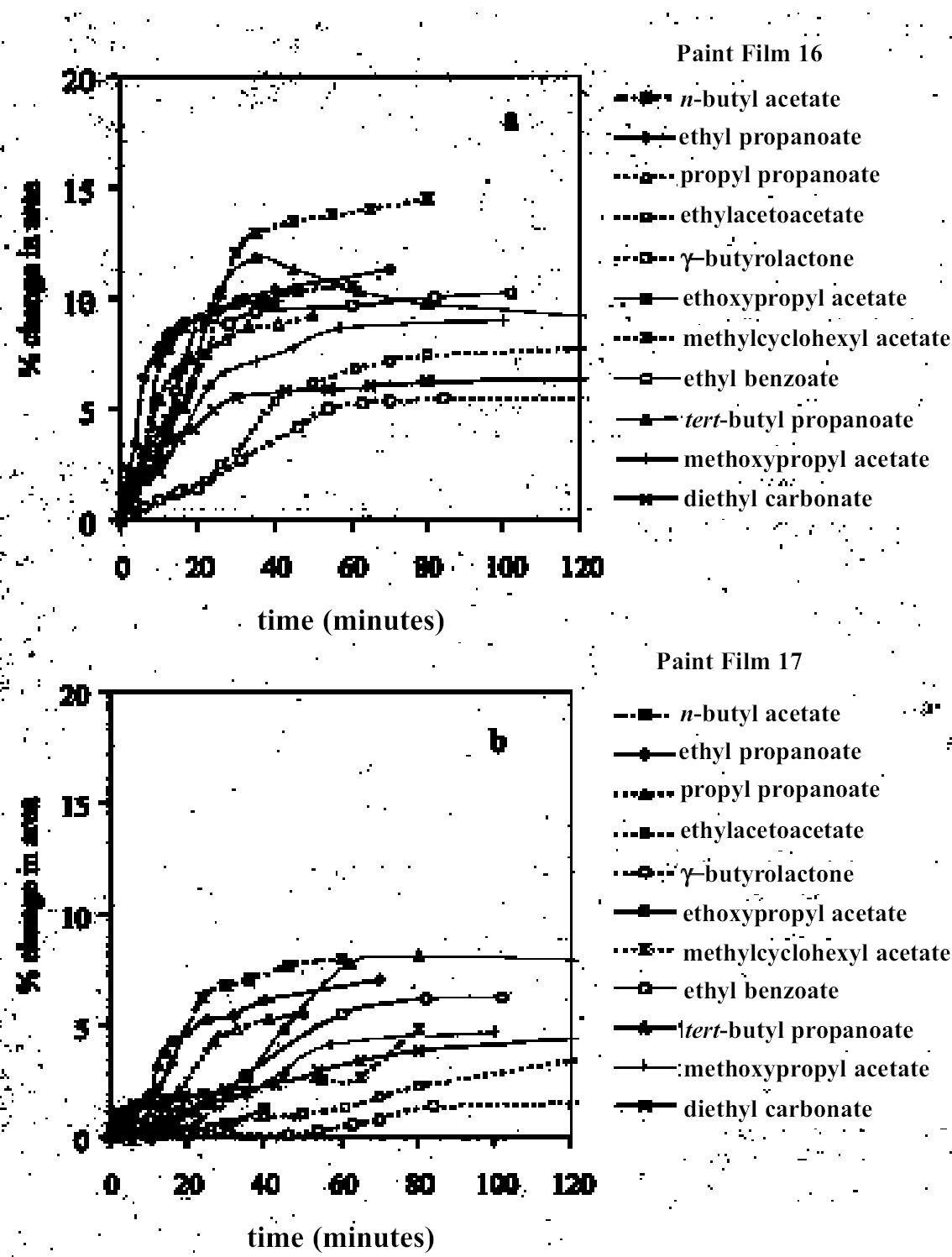
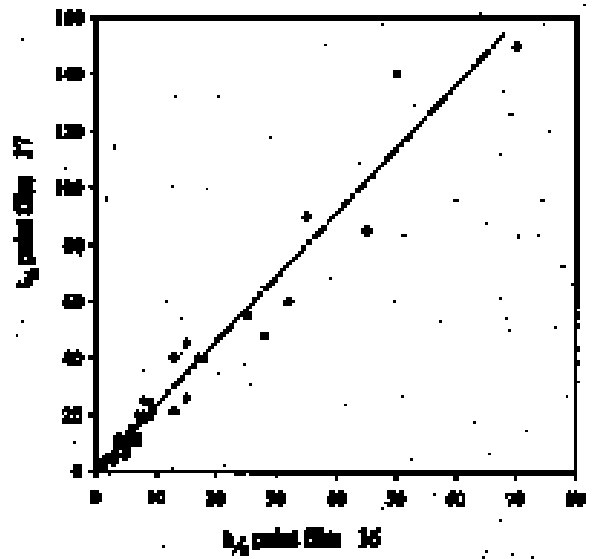
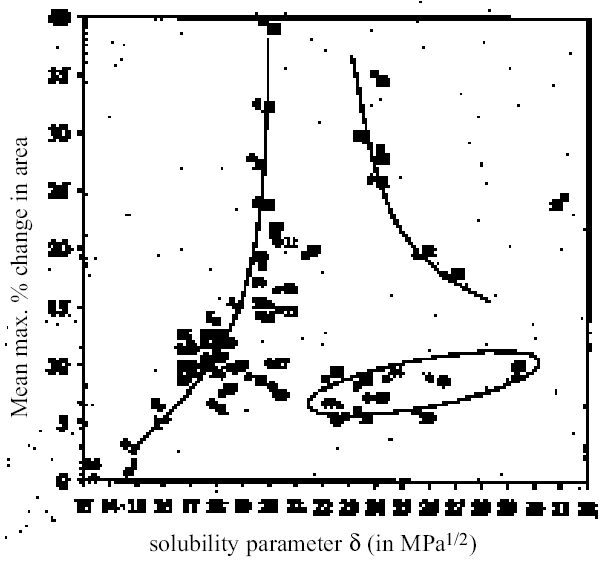
4 RESULTS AND DISCUSSION4.1 SWELLING BY INDIVIDUAL SOLVENTSRepresentative mean swelling curves for the two paint films, 16 and 17, during immersion in various organic solvents are shown in figures 2–8. The solvents are organized according to chemical class (hydrocarbons, ethers, esters, etc.). The first graph in each pair (figs. 2a, 3a, etc.) relates to paint film 16 and the second (figs. 2b, 3b, etc.) to paint film 17. Table 2 comprises, for both paint films 16 and 17, a compilation of data on the maximum values of swelling and on the rates of swelling, the latter property being defined by the time to half maximal swelling, t1/2. Table 2 contains values for various indicators of solvent polarity and serves also as the key to figures 10–11. There are many possible indicators of solvent polarity, either physical properties such as dipole moment (μ) and relative permittivity/dielectric constant (∊), or empirical parameters (Phenix 1998b). Most of the empirical parameters are derived from solvatochromic measurements. The Reichardt normalized polarity value ETN is now widely accepted as one of the more viable empirical indicators of solvent polarity (Marcus 1999). One of its principal shortcomings, however, is its apparent tendency to overestimate the polarity of any compound with an –OH group in it. The other main established solvatochromic indicator is the π* parameter of Kamlet et al. (1983). This indicator measures a blend of polarity and polarizability and was established with the intention of providing a quantitative empirical measure of the nonspecific part of van der Waals interactions, that is, contributions other than hydrogen bonding, which is covered by two other parameters, α (hydrogen bonding acidity) and β (hydrogen bonding basicity) (Laurence et al. 1994; Marcus 1999). While there is some general correlation between ETN and π*, there are some notable anomalies in the values for certain solvents, most of which are attributed to different responses to solvent polarizability effects. Comparison of the whole body of swelling data for the two paint types demonstrates that the lightaged sample, 17, generally swells more slowly and to a lower degree than 16, its unexposed equivalent. This pattern is found with virtually all solvents and is presumably due to several factors: increased paint density in the light-exposed samples, lower organic content, and, possibly, higher cross-link density. The possibility cannot be ruled out, also, that the presence of a mobile, soluble phase within the organic binder actively enhances solvent penetration in the paint binder network. Correlation of the t1/2 values for paints 16 and 17 indicates that, in general, the rate of swelling of paint 16 is of the order of twice that of 17 (fig. 9). A number of swelling curves show a marked decline in the sample area after an initial swelling peak. It is believed that this finding indicates an appreciable degree of leaching connected with swelling, as described by Stolow (1963). The absence of a distinct peak in the swelling curve does not, however, necessarily mean that leaching is not occurring to a significant extent. It is immediately apparent from the data in figures 2–8 that, as would be expected, solvents vary significantly both in the rate at which they cause swelling and in their ultimate swelling power. It appears, however, that the relationships of swelling power to solvent polarity and molecular size are rather more complex than indicated by previous analyses. Plotting the data on maximum values of swelling for paint film 16 as a function of solubility parameter ∂, as in figure 10, gives a pattern that has superficial similarities with the findings of Stolow (1976). Solvents of low-solubility parameter, such as
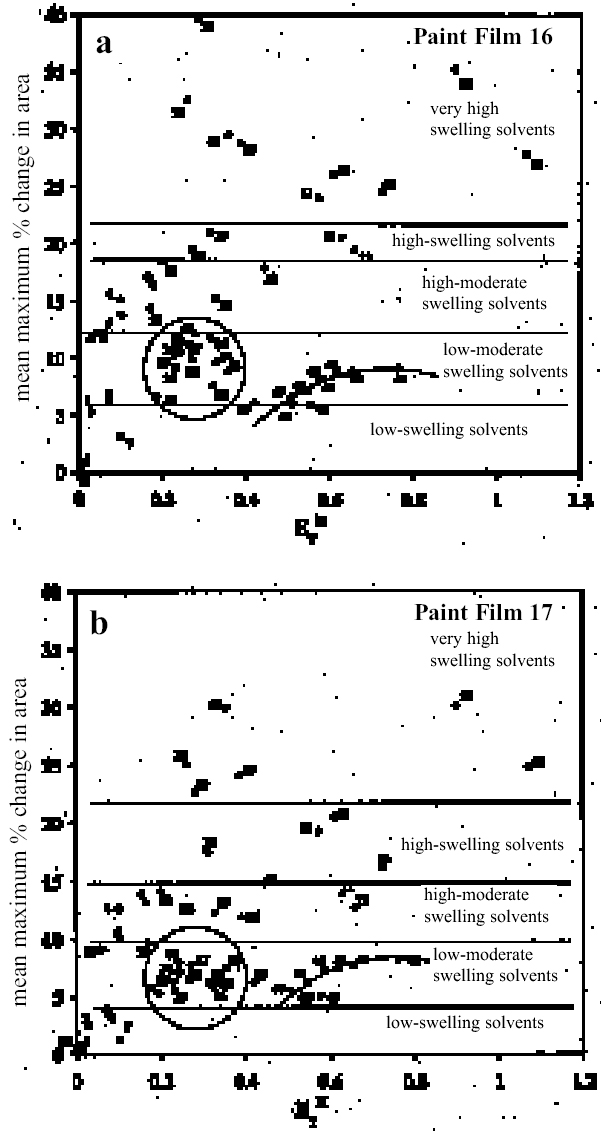
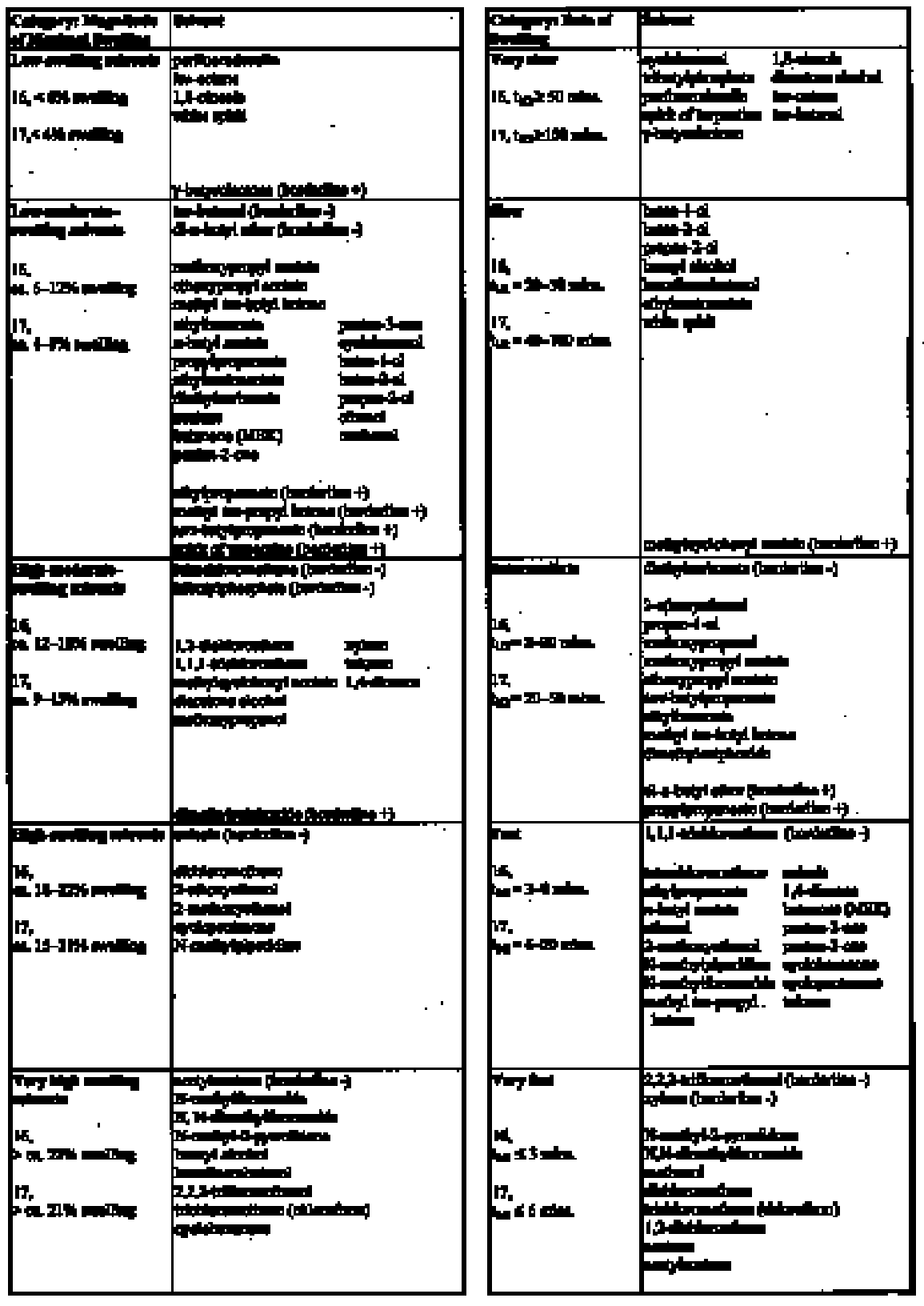
Figure 11a shows for paint film 16 the variation in maximum degree of swelling (as represented by % change in area) as a function of solvent polarity, here represented by the Reichardt normalized polarity value ETN (Reichardt 1990). Figure 11b shows the same data for paint film 17: the overall pattern of behavior is similar, although the magnitudes of maximal swelling are generally smaller. The various solvents can be placed into arbitrary categories according to their capacity to induce swelling and to their speed of action (see table 3). It can be seen that high degrees of swelling can be caused by solvents of various chemical classes over a broad range of polarity from ETN values of about 0.3 up to the supersolvents, with the highest possible values of around 1.0. A feature common to the lower polarity solvents from the group that causes high-moderate swelling or greater (tetrachloromethane, xylene, toluene, 1,1,1-trichloromethane, 1,4-dioxane, anisole, dichloromethane) is their high polarizability. Two
4.1.1 Hydrocarbons and Other Nonpolar SolventsAs might be expected from practical experience, the group of low-swelling solvents comprises mostly very nonpolar liquids, such as aliphatic hydrocarbons and related compounds (see figs. 2, 4). Perfluorodecalin, like many of its fully fluorinated relatives, is extremely nonpolar and hydrophobic. It causes barely detectable swelling in the paint films. Similar low swelling is also observed with iso-octane (2,2,4-trimethylpentane). The presence of a small proportion (<20%) of aromatics in aliphatic hydrocarbons, as in the case of white spirit, increases the swelling power of the solvent, especially on paint film 16, almost to the point of elevation to the low—moderate swelling category. For these young-mature paint films, spirit of turpentine is a low-moderate sweller. The cyclic monoterpenoid ether 1,8-cineole (eucalyptol) might also be included in the group of low-swelling, low-polarity solvents. White spirit and spirit of turpentine are rather slow in their swelling action, especially on the light-aged paint film 17: for both these solvents, equilibrium swelling is still not reached after more than 100 minutes. The aromatic hydrocarbon solvents toluene and xylene were markedly more active than the nonaromatics, causing high-moderate levels of swelling in both paint films 16 and 17, the explanation for this response probably being their greater polarizability and molecular size and shape. For these solvents the difference in magnitude of maximal swelling between the light-aged sample and the unexposed sample is relatively small. On the other hand, the nonpolar, nonaromatic solvents white spirit and spirit of turpentine show significantly lower swelling effects on the light-exposed sample 17, a finding that may reflect a shift to greater polarity caused by aging.
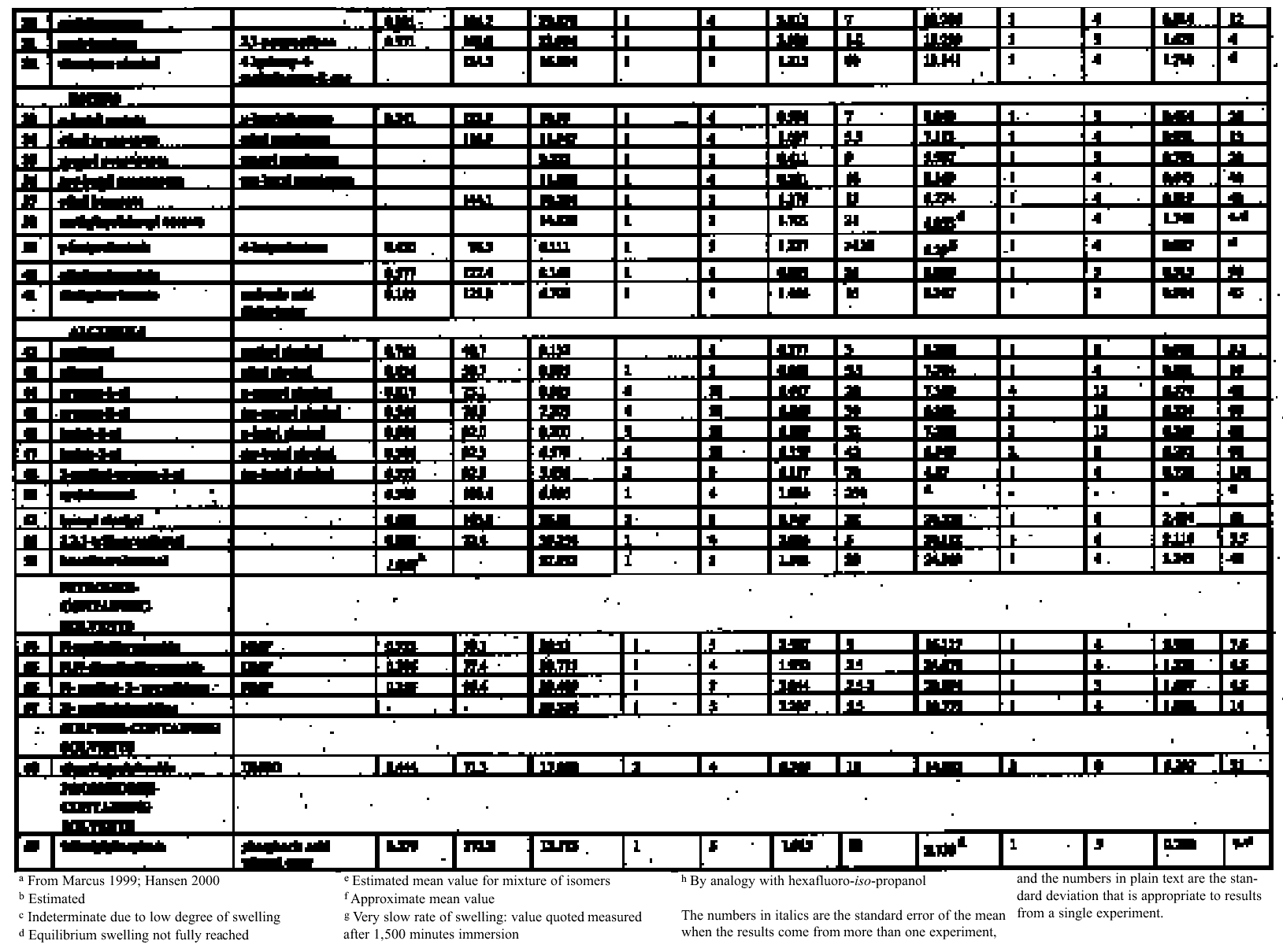
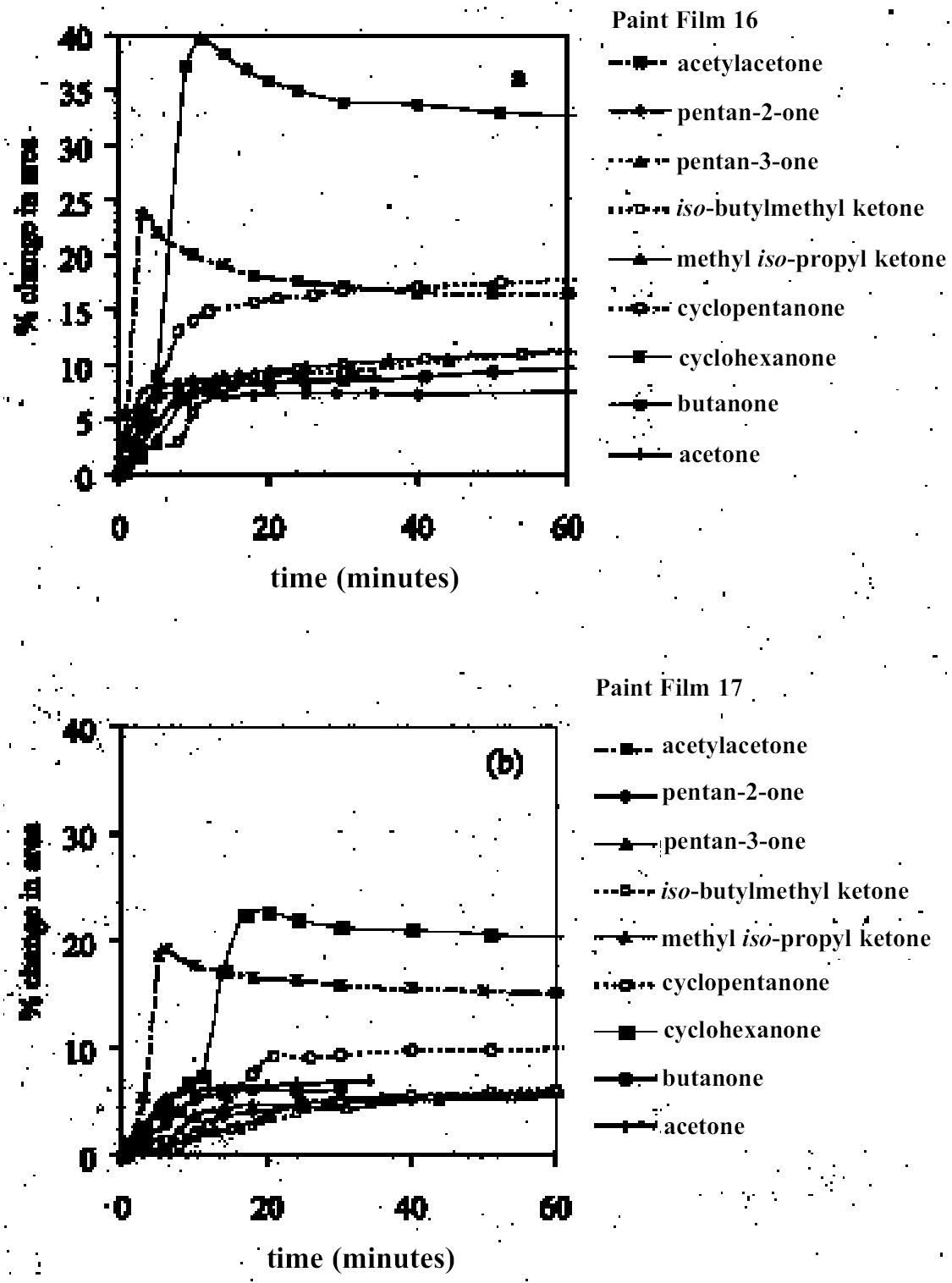
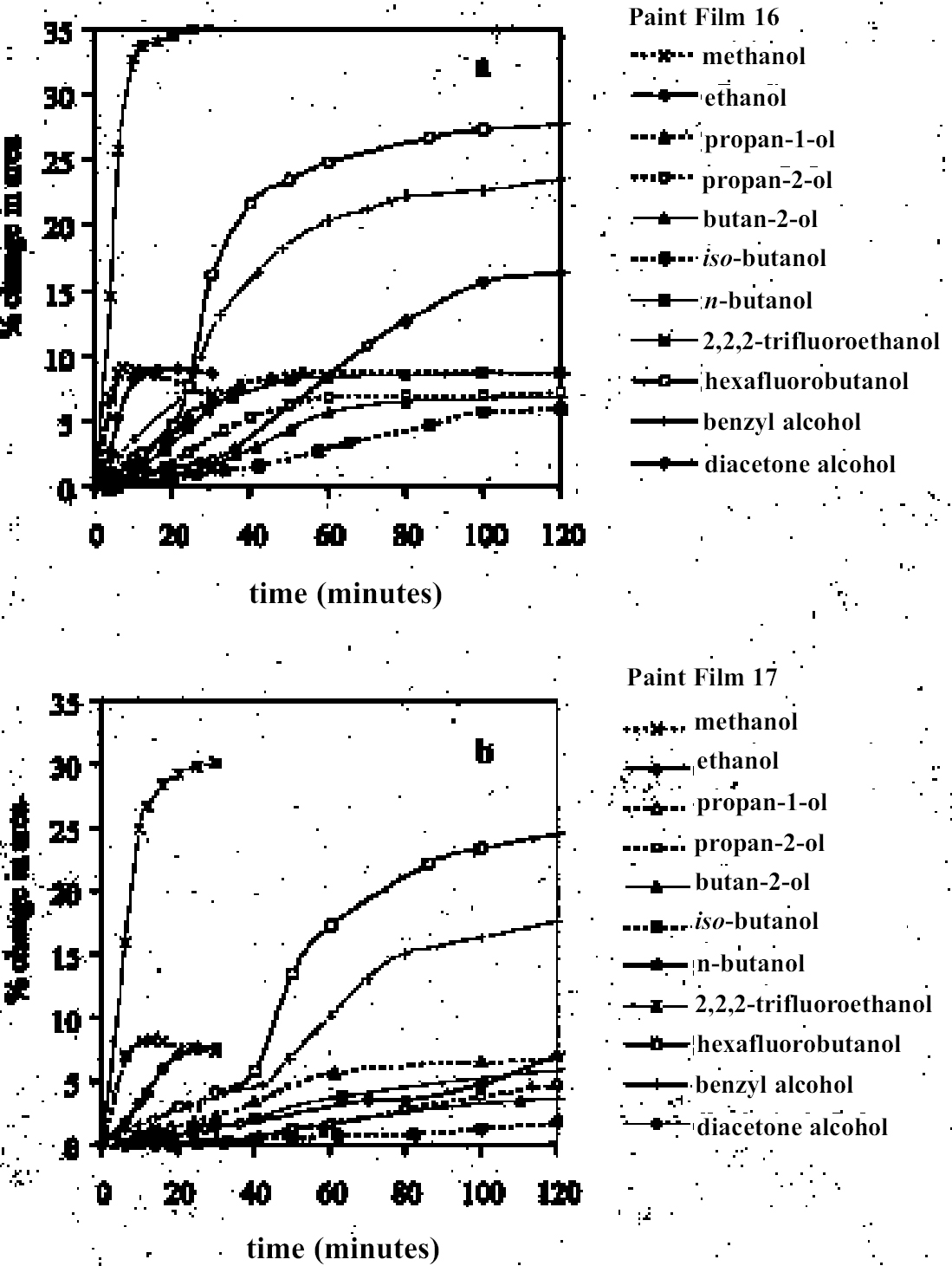
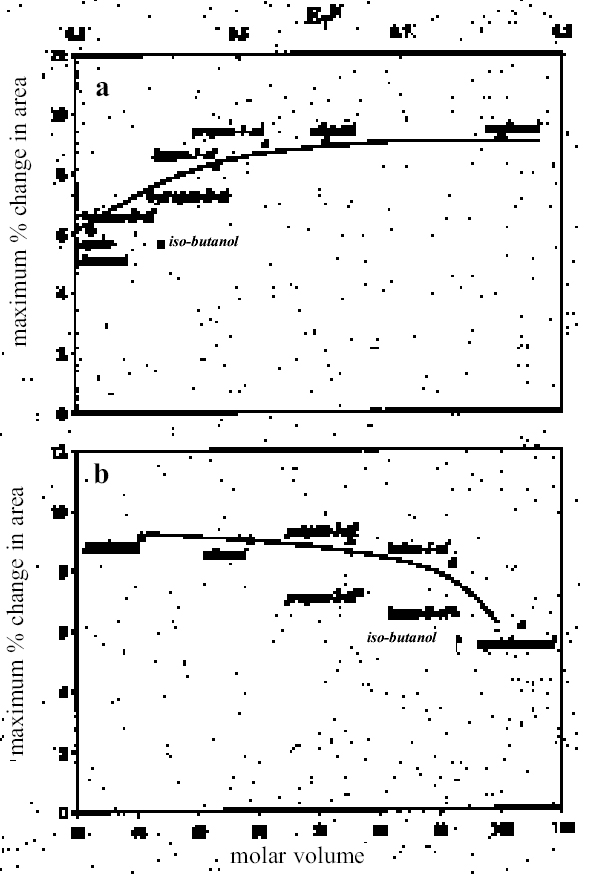
4.1.2 Ethers and Ether-AlcoholsThe mono-functional compound di-n-butyl ether (see fig. 3) falls toward the low border of the group of low-moderate swellers, most likely as a consequence of both the low polarity of this solvent and its high molecular volume. As mentioned previously, ether solvents containing additional functional groups (aromatic ring, hydroxyl, etc.) that confer greater polarity or polarizability of the molecule cause significantly higher levels of swelling, with the consequence that 1,4-dioxane and methoxypropanol fall in the group of high-moderate swellers, with anisole, 2-ethoxyethanol (cellosolve), and 2-methoxyethanol (methyl cellosolve) among the group of high-swelling solvents. In the context of solvent substitution, it is interesting to note the low-swelling power of methoxypropanol compared to 2-ethoxyethanol, with which it shares the same ether-alcohol functionality and molecular weight. Conversion of methoxypropanol to its corresponding acetate reduces the swelling power of the solvent somewhat: methoxypropyl acetate is among the group of low-moderate swelling solvents, and the same applies to ethoxypropyl acetate (see fig. 5). 4.1.3 Halogenated SolventsThe solvent properties of simple halogenated solvents (see fig. 4) are influenced predominantly by their dipolarity due to the electron-withdrawing behavior of the halogen atom(s). Carbon tetrachloride and chloroform are well known as good solvents for fatty acids and fatty acid esters (Schmid 1973), and it is not surprising that solvents of this class are found among the most active on these young-mature paint films. For the group of chlorinated solvents examined here, swelling power is related, among other things, to polarity and molar volume. As found by Stolow (1976), chloroform (trichloromethane) is one of the most powerful solvents in its swelling action, producing very high levels of swelling, >25% for both paints 16 and 17, followed by dichloromethane. Both these solvents are aprotic dipolar compounds with small molar volumes. The reason for the particularly strong swelling power of chloroform is unclear, but it may be related to its hydrogen bonding—donor behavior in addition to its dispersion force and dipolar interactions. As far as rate of swelling is concerned, all of the halogenated solvents tested here, with the exception of perfluorodecalin, fall into the categories of “fast” or “very fast” acting liquids. 4.1.4 Esters and Related SolventsAs noted earlier, as a class, aliphatic ester solvents (see fig. 5) all generally fall in the category of low-moderate swellers, producing maximum changes of area 6–12% in paint film 16. There are a few exceptions to this general classification: ethyl propanoate, tert-butyl propanoate, and tributyl phosphate (data plotted in fig. 8), all of which are borderline high-moderate swellers for paint film 16, and methyl-cyclohexyl acetate. Increased dispersion force interactions deriving from the greater compactness of the cyclic ester may contribute to the enhanced swelling power of methylcyclohexyl acetate compared to acyclic aliphatic esters. For paint film 17, all the aliphatic esters fall comfortably within the low-moderate swelling category. Indeed, it is useful to note the similarity in swelling power of the various acyclic aliphatic esters and even ethyl benzoate. However, their rates of swelling vary considerably, essentially in relation to molecular size. Other solvents related to the ester group— diethylcarbonate and ethylacetoacetate—also produce maximal levels of swelling in the low-moderate category; and the cyclic ester γ-butyro-lactone is on the border with the group of low-swelling solvents. These last two solvents are particularly interesting, as they appear to behave similarly to the aliphatic alcohols in that they are quite strongly polar liquids, yet they produce, in the order of things, only low swelling. γ-butyrolactone is a very slow-swelling solvent that is distinctive in that the degree of swelling it causes respectively in paints 16 and 17 is very similar. 4.1.5 Ketones and Related SolventsPerhaps the most interesting aspect of the results for the aliphatic ketones (see fig. 6) is the diversity of All of the acyclic ketones (acetone, butanone, pentan-2-one, etc.) produce roughly similar degrees of maximal swelling (8–12% change in area for paint film 16). The classification of acetone in terms of swelling power should be noted. This solvent is often referred to as a “strong” solvent for oil paint, and, while this label may legitimately apply to its capacity to extract soluble compounds, as far as swelling is concerned acetone is firmly in the “low-moderate” category. As happens in a number of cases with other solvent types, the introduction of additional functional groups changes (usually by increasing) the swelling properties of ketone solvents. Accordingly, acetylacetone is on the border between the groups of high-and very high swelling solvents. Acetylacetone and acetone are among the fastest-acting solvents, with t1/2 values of less than two minutes and four minutes for paint films 16 and 17, respectively. The cyclic ketones swell at a faster rate than analogous acyclic compounds, probably by virtue of their smaller molar volumes. 4.1.6 Alcohols and Related SolventsAs mentioned previously, it can be seen from both figures 10 and 11 that the simple aliphatic alcohols, including cyclohexanol, form a distinct group of solvents characterized by high polarity and low-moderate swelling power (see fig. 7). Looking at the data for paint film 16 in more detail, it can be seen that the capacity to induce swelling increases with increasing polarity (fig. 12a) and with decreasing molecular size/molar volume. The finding that, for these plain linseed oil samples, the smaller, more polar alcohols (methanol, ethanol) are capable of producing greater degrees of swelling than the linear three-and four-carbon alcohols is at variance with the results of Stolow (1976) for linseed stand oil films. This finding
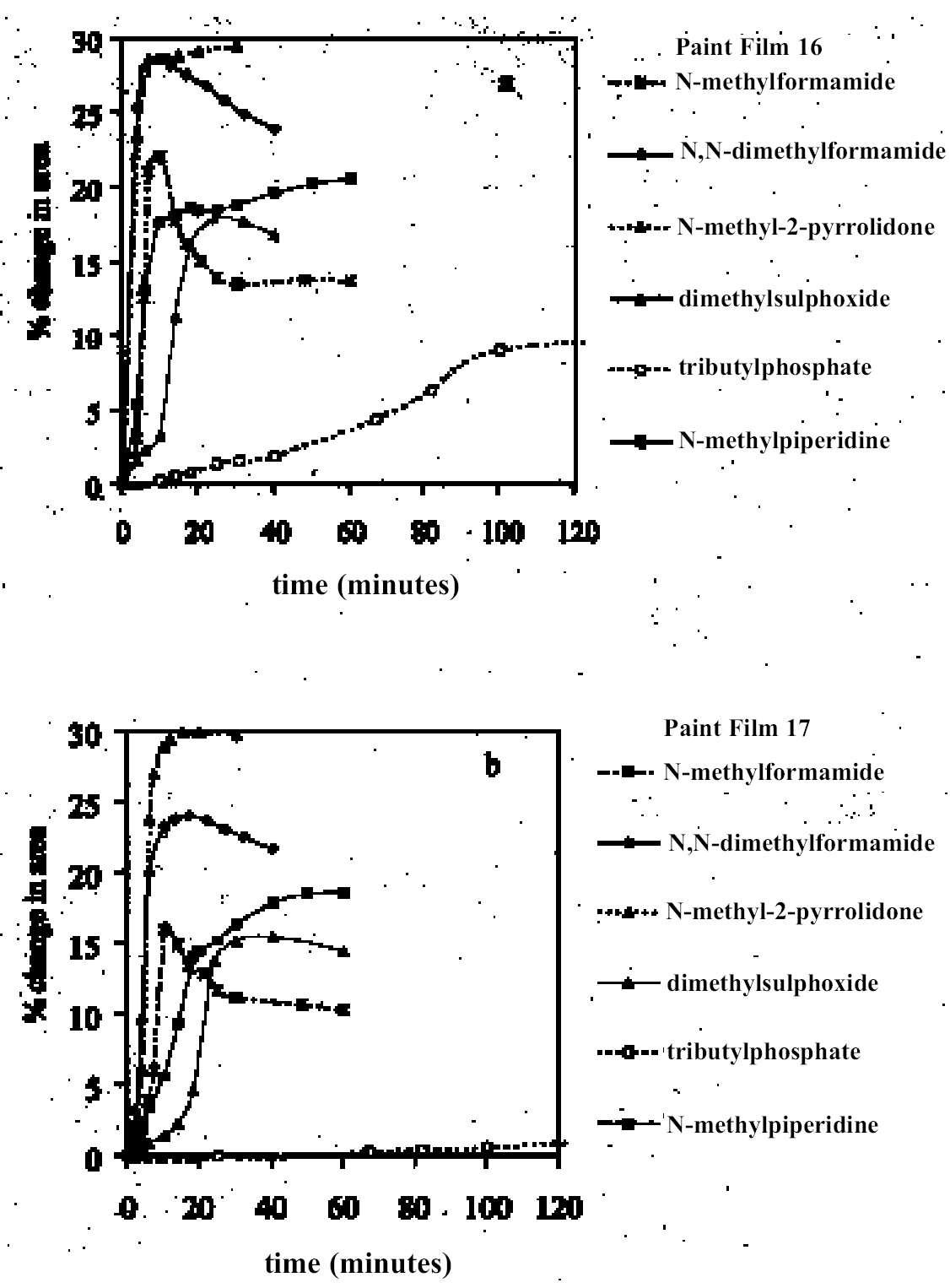
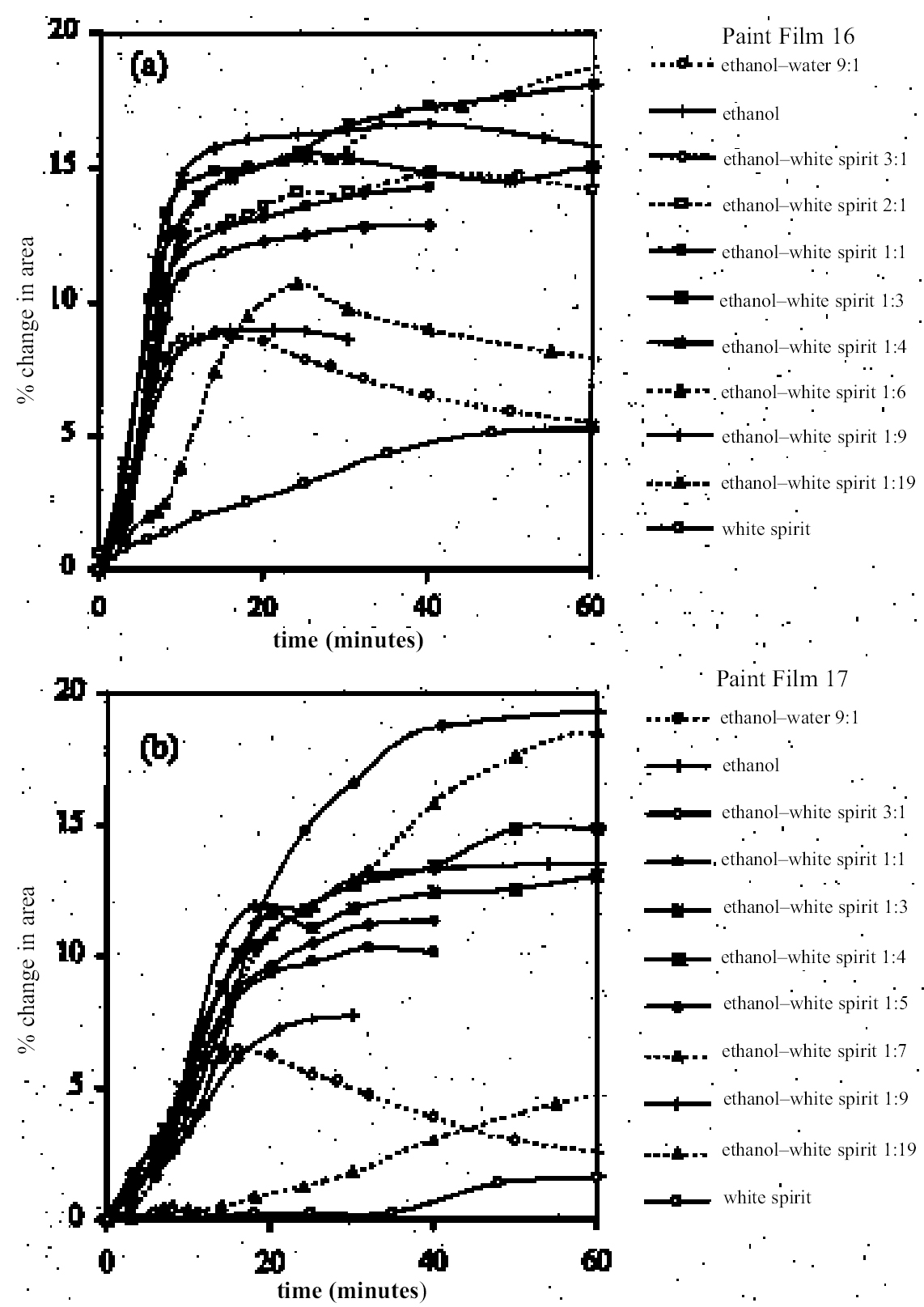
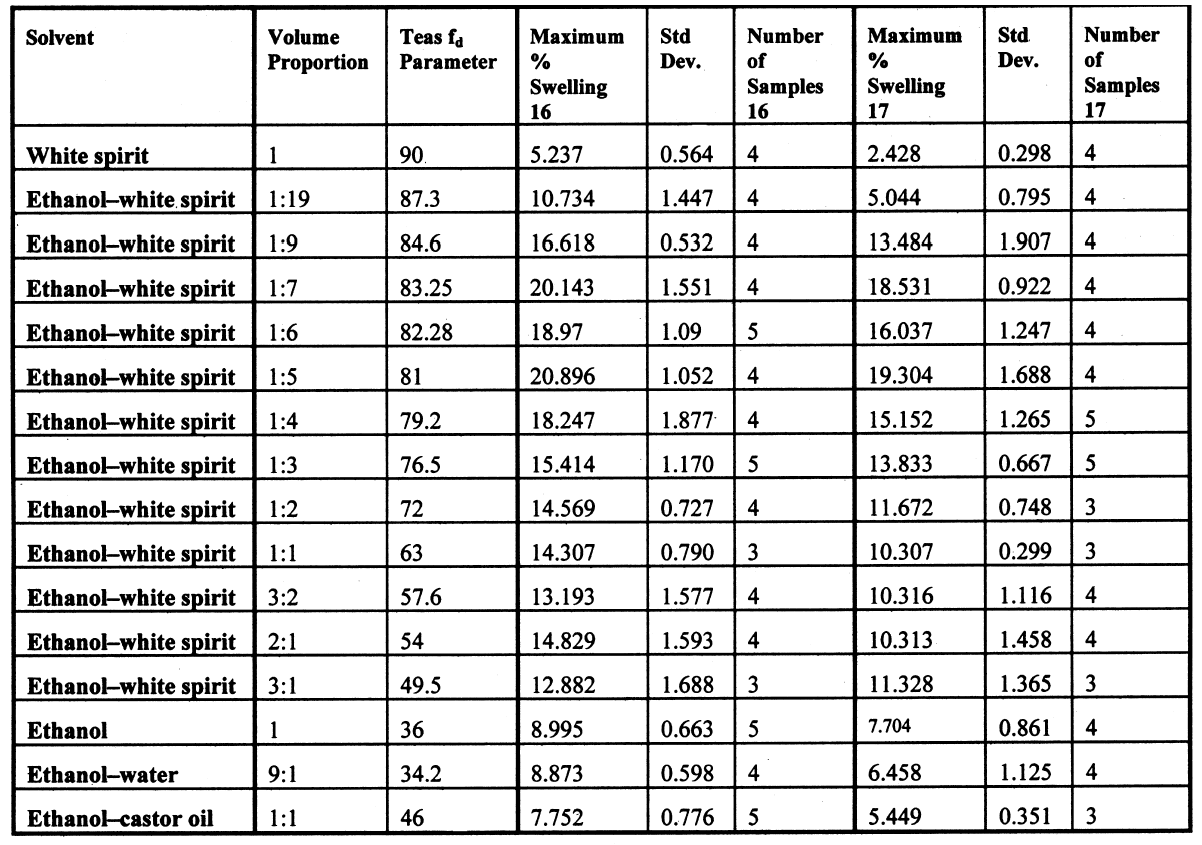
As in the case of ketones, alcohol solvents with additional functional groups have different (again, mostly greater) swelling properties than the simple alcohols. The keto-alcohol, diacetone alcohol, is a high-moderate sweller, albeit very slow in its action. Benzyl alcohol—widely used now as an additive to aqueous cleaning formulations to enhance the activity on drying oil—containing coatings—is, not surprisingly, in the class of high-swelling solvents. The greatest degrees of swelling among the alcohols are caused by the two fluorinated alcohols, hexafluorobutanol and 2,2,2-trifluoroethanol. These are among the most polar organic compounds that are known and fall into the group of most active solvents on oil paint, comparable with chloroform, cyclo-hexanone, and dimethylformamide. 4.1.7 Nitrogen-, Sulphur-and Phosphorus-Containing SolventsCompounds from this group (see fig. 8) are also widely recognized as some of the most powerful organic solvents that exist, and, in essence, this char-acteristic derives from their ability to engage in almost the full range of intermolecular interactions with solute molecules. The amides, for example, three of which were examined here (N-methylformamide, N, N-dimethylformamide, and N-methyl-2-pyrroli-done), are all strongly dipolar solvents, as is dimethylsulphoxide: all are characterized by high dipole moments, dielectric constant, and polarity parameters. The very high value of ∂ for N-methylformamide (NMF, solvent 54) reflects the very high specific cohesiveness of this liquid, which arises in part from its strong intermolecular association. Like water, N-methylformamide is classed in the group of hydrogen-bonded strongly associated solvents (HBSA) (Reichardt 1990). It is not surprising, therefore, that these solvents cause high levels of swelling in the paint films under test, a finding that is consistent with empirical wisdom from conservation practice. Dimethylfor-mamide (DMF), after all, is resorted to for extreme problems such as the regeneration of paint films severely blanched by exposure to water. With paint type 16 the amides all produce roughly similar degrees of maximal swelling (25–30% change in area, “very high” category), with the cyclic compound N-methylpyrrolidone (NMP) being the most active despite having the largest molar volume. The high degrees of contraction observed in the samples during immersion in NMF and DMF possibly indicate substantial leaching of soluble material from the swollen paint. For paint film 17, the swelling power of N-methylformamide is apparently diminished in comparison to DMF and, especially, NMP. This last solvent has the most powerful effect on the light-aged paint film of all solvents tested. All three amides are fast in their swelling action, with t1/2 values for paints 16 and 17 of ≤ 5 minutes and ≤ 7.5 minutes, respectively. Unusually, the rate of swelling of NMF is slower than the larger molecule DMF. The cyclic amine N-methylpiperidine was included in the range of test solvents essentially for comparison with the cyclic amide NMP: they have similar structures save that N-methylpiperidine has an additional carbon atom in the ring and lacks the carbonyl group. While still a member of the high-swelling group of solvents, N-methylpiperidine has somewhat lower swelling power than NMP, possibly due to diminished hydrogen-bonding interactions. Dimethylsulphoxide (DMSO), rather surprisingly, does not match the nitrogen-containing solvents for swelling power, being on the border between the high-moderate and high swelling groups. The rate of swelling in DMSO is also low by comparison with the amides, especially for the light-aged paint film 17. Tributylphosphate is one of the slowest acting of all the solvents tested, a characteristic that is almost 4.2 SWELLING BY BINARY SOLVENT MIXTURES CONTAINING ETHANOLBinary mixtures of a polar solvent, such as ethanol, and an apolar solvent, such as white spirit or iso-octane, are commonly used in varnish removal from paintings. One of the most important findings of Stolow's research was that such mixtures could induce a degree of swelling that was significantly greater than that of either of the pure solvents used alone (Stolow 1963; Feller 1976). Since binary solvent mixtures containing ethanol are such an important element of the conservator's range of cleaning tools, the swelling responses of paint films 16 and 17 were measured for a range of mixtures across the whole spectrum of polarity, from 100% white spirit to 100% ethanol and beyond. Details of the various solvent mixtures tested are given in table 4. The grade of white spirit used was Stoddard solvent (BDH Merck Ltd.). Following the approach of Feller (1976), the solvent power of the various mixtures is described by the Teas fractional dispersion parameter fd. Figure 13 shows the swelling curves for paint films 16 and 17 immersed in selected mixtures from the range tested. Selected early data points have been excluded to aid clarity of graphic presentation. It can be seen immediately that mixtures of the two solvents produce considerably higher degrees of maximum swelling than either of the pure solvents. Interestingly, for the binary mixtures of ethanol and white spirit containing 10% or more ethanol, the initial rate of swelling (up to ca. t = 8–10 minutes) is effectively the same and comparable to that for pure ethanol. This result might suggest that the rate of swelling, and probably also the rate of penetration, caused by a binary solvent mixture is governed by the diffusion of the faster component. Figure 14 shows, for both paint films 16 and 17, the maximum values of swelling caused by binary solvent mixtures containing ethanol in relation to their Teas fractional dispersion parameters, fd. Some of the mixtures containing larger proportions of white spirit produce surprisingly high levels of swelling (>20% for paint film 16, >18% for paint film 17) putting them almost into the category of high swellers. The high sensitivity of both paints, especially 16, to solvent mixtures containing as low as 10% ethanol is perhaps an indication of their relatively youthful nature. The greatest degrees of swelling are caused by solvent mixtures in the range fd 77–85: this peak may have some correspondence with the single peak observed by Stolow for lead white—stand oil films (Stolow 1963, fig. 4) However, there remains a broad plateau representing mixtures of higher polarity that cause swelling to levels comparable with the high-moderate category described previously. Pure ethanol and a 10% water-ethanol mixture, by comparison, are low-moderate swellers, and pure white spirit is a low sweller. This pattern suggests that, while young-mature oil paint films may show a particular sensitivity to a narrow window of relatively apolar solvent mixtures, they are also capable of being swollen to a significant degree by solvent mixtures covering a much broader polarity range. As observed with virtually all pure solvents, the light-aged paint film 17 swells to a lower degree than its unexposed analog, but the general pattern of behavior is similar, although for paint 17 there are signs of reduced sensitivity to very apolar solvents, white spirit and white spirit: ethanol 19:1. It was noted by Michalski (1990) that mixtures of ethanol and castor oil might provide a useful means of skirting round the peak swelling region of oil paint, by virtue of the distinctive solubility parameters (fd: 56, fp: 11, fh: 33) of the oil. Only one such mixture has been tested to date, castor oil: ethanol 1:1, and while this mixture appears to produce slightly lower maximal degrees of swelling than ethanol—white spirit mixtures of comparable polarity, the difference is only slight. It remains to explore the swelling power of mixtures of ethanol and castor oil in the more significant region of lower polarity.
|