THE SWELLING OF ARTISTS' PAINTS IN ORGANIC SOLVENTS. PART 2, COMPARATIVE SWELLING POWERS OF SELECTED ORGANIC SOLVENTS AND SOLVENT MIXTURESALAN PHENIX
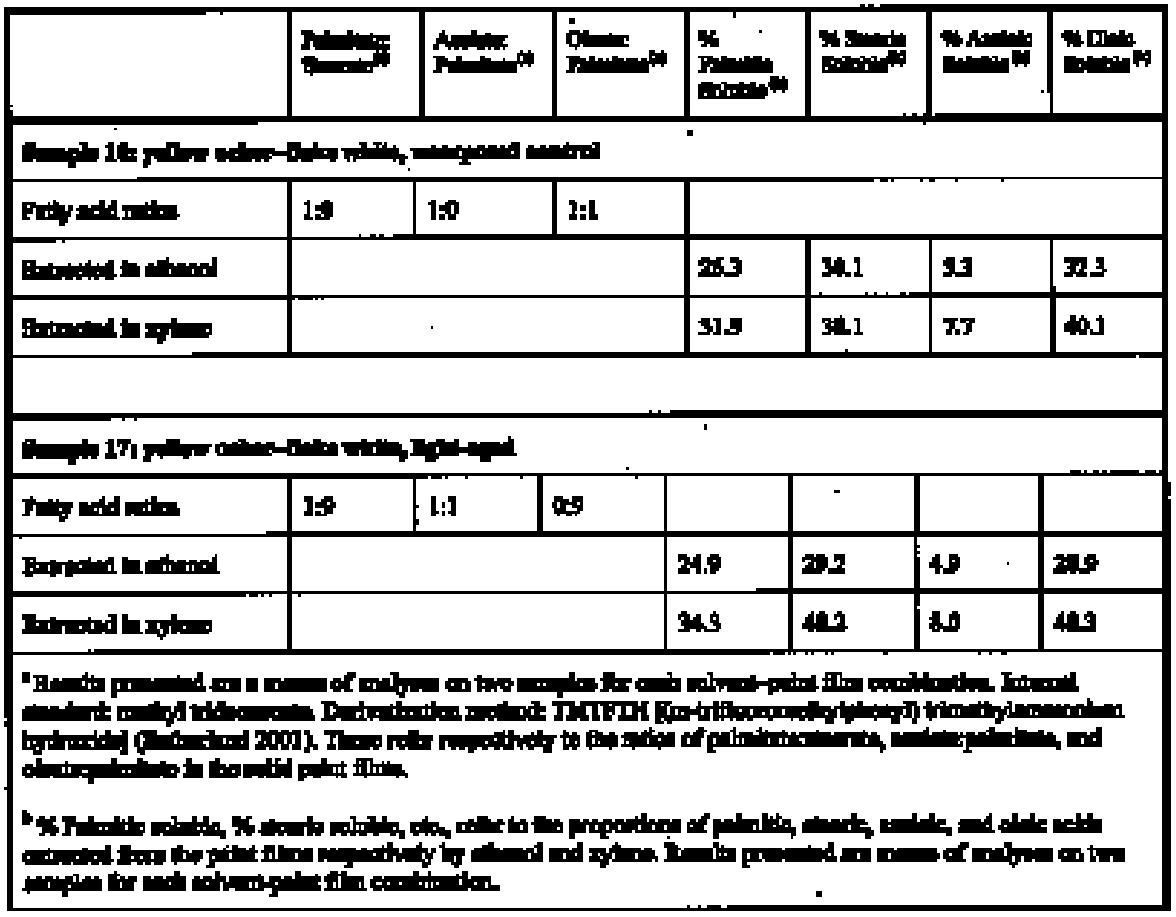
3 EXPERIMENTAL METHODThe method used for measuring the swelling of artist's paints during immersion in solvents is that reported elsewhere in this journal (Phenix 2002). 3.1 PAINT FILMSThe results reported here relate to just two paint films from the group described in Phenix (2002), namely samples 16 and 17. These are paint films prepared in 1991 essentially from proprietary oil paints (Winsor & Newton Artists' Oil Colours), with added dry pigment to increase the pigment volume concentration. They are composed identically as follows: yellow ocher oil paint (3 parts by weight) + flake white oil paint (3 parts by weight) + yellow ocher dry pigment (1 part by weight) + flake white dry pigment (1 part by weight). The paints were cast as uniform films on polyester film and allowed to dry for several weeks. Both sets of samples 16 and 17 were then housed in a purpose-built light-aging apparatus for approximately 18 months (Phenix 2002). Paint film 17 was exposed to UV-filtered daylight (estimated at 50–70 Mlux hours), while 16 was not exposed to light, being completely covered with aluminium foil for the period in the light-aging chamber. Paint films 16 and 17 are, therefore, identical except that 17 has had a substantial period of light exposure. They are now 140 μm and 130 μm thick respectively. Organic chemical analysis by gas chromatography– mass spectroscopy was conducted to characterize the nature of the paint samples and of soluble, extractable components (Sutherland 2000). The analyses were conducted following protocols established specifically for the quantification of organic compounds extractable from oil paints (Sutherland 2001, 89, 141–57). A summary of results is compiled in table 1. Both paint films 16 and 17 show a high proportion of oleic acid, indicating that, even though 17 has been exposed to light-aging, drying/oxidation has not progressed very far. These might be classed as youngmature paint films. The palmitate: stearate (P:S) ratios of 1:9 are typical for linseed oil. The azelate: palmitate (A:P) ratios differ slightly, with the light-aged sample Interestingly, both paint films were shown by analysis to have relatively low overall solubility. In fact, these samples were originally selected for intensive study partly because they tended to produce less variable results than other paint films on account of their lower sensitivity to leaching. The low abundance of soluble components compared to other young paint films probably derives from the period in the aging apparatus where elevated temperatures (possibly exceeding 40�C) will have encouraged volatilization of low molecular weight species (Erhardt et al. 2000). The two paint films are more or less similar in terms of solubility: the additional lightaging experienced by 17 appears to have had only minor effect. The greater solubility of both samples in xylene compared to ethanol is slightly unusual (Sutherland 2000).
As reported previously, the method employed here allows the swelling of several paint samples to be measured simultaneously. Results for a group of samples of the same type in any given solvent are combined to produce a mean swelling curve. The sample sets from which the mean swelling curves are derived vary in size depending on the variability of results and the number of repeat experiments required to reduce the standard error to an acceptable level.1 For the results reported here, the number of 3.2 SOLVENTSFor the most part, the test liquids used in this study came from newly purchased batches of analytical grade or general-purpose grade solvent. In a few instances, swelling experiments were carried out using existing stock available at the Courtauld Institute. |