THE SWELLING OF ARTISTS' PAINTS IN ORGANIC SOLVENTS. PART 2, COMPARATIVE SWELLING POWERS OF SELECTED ORGANIC SOLVENTS AND SOLVENT MIXTURESALAN PHENIX
5 SUMMARYIt has been shown that the low-power microscopy—image analysis technique can be used successfully for measuring the comparative swelling powers of organic liquids on young-mature oil paint films such as the ones tested here. The results obtained in this study indicate a more complex relationship between swelling power and individual solvent characteristics, especially polarity, than is reflected by previous studies. Several types of polar solvent are capable of generating high degree of swelling in the paints tested. Although the lower alcohols produced relatively low degrees of swelling in comparison with other solvents having high values of Reichardt polarity ETN and accordingly were classified as low-moderate swellers, methanol and ethanol in particular have been shown to be appreciably more active on these test paints than on the lead
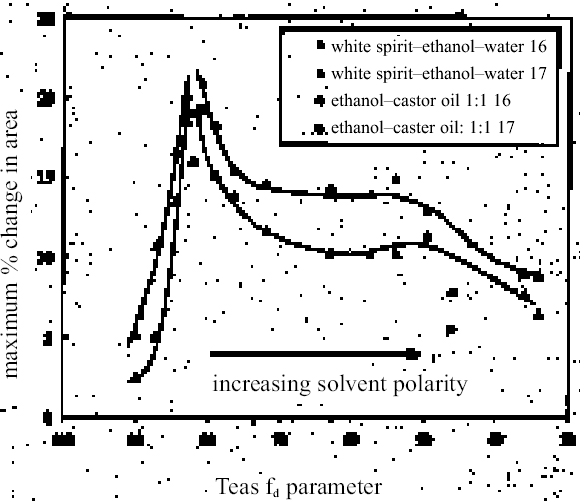
In the vast majority of cases, the light-aged paint film 17 shows distinctly lower degrees and slower rates of swelling than its unexposed equivalent. It is conceivable that more severe aging would lead to progressive decline in these properties. The markedly lower sensitivity of paint film 17 to some apolar solvents, such as white spirit and di-n-butyl ether, may point to contraction of the swelling region on the apolar side caused by light exposure. Paint film 17 remains similarly sensitive to solvents at the more polar end of the range as its unexposed equivalent, 16. It can be seen that there is not a very clear relationship between the swelling power of a given solvent and a single descriptor of solvent power such as Reichardt ETN or total solubility parameter, ∂. The plots in figures 10 and 11 show, at best, rather loose patterns of behavior, and this finding applies also with most other polarity indicators. It seems likely that attempting to correlate swelling power against any single indicator of solvent power will lead to similarly unresolved outcomes, as many factors will influence the manner of paint solvent interaction. As well as polarity, other factors that will be significant are molecular size, polarizability, hydrogen-bonding donor and acceptor properties, electron pair donor/acceptor behavior, and acidity/basicity. An approach to data analysis that addresses the potential contribution of many factors, such as multivariate analysis, may ultimately be more fruitful. The finding of swelling powers almost reaching the “high” category for some of the ethanol—white spirit mixtures in the range of Teas fd 80–85 has significant implications for cleaning practice, particularly for relatively young paintings. On the young-mature paint films tested here, the swelling power of white spirit mixtures increases quite significantly as the proportion of ethanol is increased above about 8–10%. The sharp increase in paint sensitivity in this relatively low polarity territory may give the conservator using the conventional practice of progressive addition of ethanol to white spirit to increase polarity very little room to maneuver in finding a solvent mixture that will effectively remove a varnish yet still be safe for the underlying original paint. It may be that, in such cases of removing a varnish from a relatively apolar, young-mature paint film, working from the other end of the polarity range may be a more effective strategy. Passages in many paintings from the later part of the 19th century and the first half of the 20th century may be of this type. In terms of practical application of the results presented here, the main thing has been to clarify the activity of the lower alcohols and of other solvents commonly used in conservation, yet previously untested. Thus the powerful action of strongly dipolar solvents such as N, N-dimethylformamide and N-methyl-2-pyrrolidone—used variously to help remove oil-containing varnishes and overpaints— have been confirmed. Similarly, other solvents occasionally resorted to by conservators to remove tough coatings containing oil (e.g., cellosolve, benzyl alcohol, diacetone alcohol, and dichloromethane) have also been shown to be within the “high-moderate,” “high,” and “very high” swelling categories. Although, as mentioned previously, the lower aliphatic alcohols have been found to be stronger swellers for oil paints than perhaps reflected by Stolow's (1976) stand oil data, the simple alcohols (and acetone) are mostly reassuringly low-moderate swellers. Considering also their polarity and their health and safety properties, the value of the alcohols as favored tools in the solvent-cleaning of paintings remains uncompromised by the present findings. Other members of the group of low-swelling, high-polarity solvents have been identified, however, as perhaps warranting further investigation: ethylacetoac-etate and γ-butyrolactone. It is envisaged that the data presented here, in addition to helping the practical conservator anticipate and control risk in varnish removal, may also serve to guide formulation of agents for the active removal of paint, as in the cases of removing overpaint from paintings or from, say, historic decorated interiors. |