THE SWELLING OF ARTISTS' PAINTS IN ORGANIC SOLVENTS. PART 2, COMPARATIVE SWELLING POWERS OF SELECTED ORGANIC SOLVENTS AND SOLVENT MIXTURESALAN PHENIX
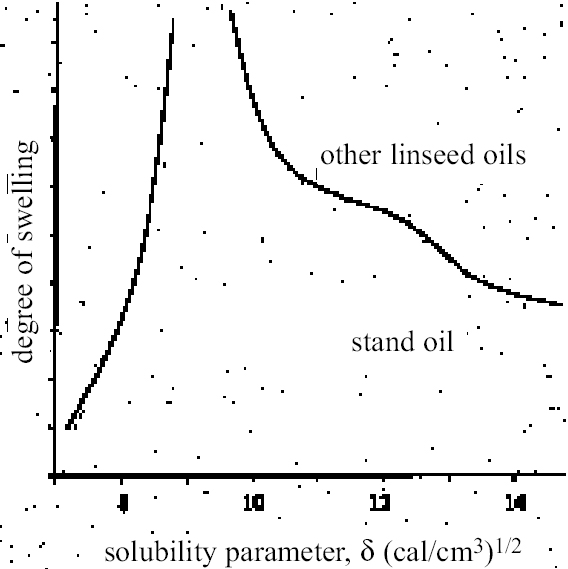
2 REASSESSING THE HEDLEYSTOLOW PEAK SWELLING REGIONAs Ruhemann (1968) clearly recognized, there are difficulties in correlating the results of experimental swelling studies with the practice of cleaning old paintings. These difficulties derive from the nature of paint films that have been tested. All the available swelling data relate to young paint films, less than 25 years old: and, within conservation, swelling data on paint films composed of linseed stand oil have been presented most widely. Such films are likely to be significantly different in chemical character from those of old paintings. Both the comparatively young age and the choice of stand oil as binder suggest that existing data may significantly underestimate the response of old paint films to solvents at the more polar end of the spectrum, particularly to those that are strongly dipolar or engage in hydrogen bonding. Dried films of stand oil are known to be considerably less oxygenated than those of blown or boiled oils or simple linseed oil dried normally in air (Mills and White 1999). For example, according to Stolow's data on the lead white—stand oil films, ethanol is a distinctly low swelling solvent comparable in effect with the aliphatic hydrocarbons. However, the practical conservator learns very quickly that, in the order of things, ethanol can have a moderately strong effect on paint. It might be expected that the swelling regions of old oil paint films are shifted toward the direction of higher polarity. Practical experience of cleaning also suggests that, in many cases, paint films can be vulnerable to solvents and solvent mixtures over a much broader range than indicated by the Hedley-Stolow peak swelling region, particularly on the polar side. By basing his peak swelling region only on Stolow's lead white-stand oil swelling data (Feller et al. 1971), Hedley inadvertently carried this limitation over into his Teas chart treatment. While acknowledging its continuing value as a guide to solvent usage in cleaning, we must now perhaps face the fact that Hedley's solubility parameter framework is constrained by its reliance on young stand oil paint films as the model of oil paint behavior. There is strong evidence that this kind of film has distinctive properties. Stolow, for example, also presented data on the swelling of other linseed oil film types as a function of solvent solubility parameter, ∂ (Stolow 1955, 199–200a; 1957, 399). What these plots reveal is the anomalous behavior of the stand oil film: all the other films show significant degrees of swelling caused by the polar solvents including the alcohols. Stolow accepted that “on the whole, stand oil films behaved somewhat differently from non-stand oil films” (Stolow 1955, 201). The different swelling behavior of stand oil compared to other linseed oil films is shown schematically in figure 1. These differences were attributed to the solubility parameter properties of the solvents rather than to the internal chemistry of the paint films. The attraction of the swelling data on the stand oil paint films may have been the relative symmetry in the curves of swelling vs. ∂, which complied with the idea of a Gaussian function (Stolow 1955, 198). Also interesting in Stolow's swelling data for n-alcohols with stand oil is the variation in degree of swelling with molecular size: the larger the molecule, the greater degree of swelling measured, a finding that is rather at odds with conventional wisdom that, all other things being equal, smaller, more compact molecules cause greater degrees of swelling (Hansen 2000, 19). The lower-swelling power of iso-and sec-alcohols compared to corresponding n-isomers is also indicated in Stolow's research. The high-swelling effects known by practical conservators of strongly dipolar solvents, such as
There are other reasons to believe that old oil paint films may be more strongly affected by polar solvents than implied by the data of Stolow (1976) or by the interpretation of Hedley (1980), and these derive from recent advances in understanding of the chemistry of deterioration processes in oil paints. There is now good evidence to suggest that real, old paint films are significantly different in their internal chemistry from the young paint films that have formed the basis of most scientific studies of the cleaning process. The methodological problems this evidence has presented in relation to scientific investigations of cleaning have been discussed elsewhere (Phenix 1998a). That oil paint films become more polar on aging, and consequently become increasingly sensitive to polar solvents, including water, has been recognized for many years (Frilette 1946; Elm 1949). Explaining the increased tendency of old (oil-modified alkyd) paint films to be plasticized by water, Brunt (1962) noted that “the oxidative drying process results in the building of a large number of hydrophilic groups because the acid number greatly increases. It is very probable that these polar groups are responsible for the sensitivity to water. A small quantity of water gives much plastification.” Oxidation is not the only aging process that may contribute to the increased polarity of the organic phase of old oil paint films. Recent research on curing and aging of oil paint films points to hydrolysis (or de-esterification) of the glyceride ester polymer network also as possibly a significant factor in the chemical alteration of oil paint binder due to aging (Schilling et al. 1997, 1999; van den Berg et al. 1999; Erhardt et al. 2000). If this is the case, then de-esterification/hydrolysis would result in quite marked changes in the internal cohesive chemistry of paint films. De-esterification/hydrolysis of the cross-linked polyester network of a dried, cured oil paint film leads to the formation of a residual cross-linked polymeric fraction that contains carboxylate groups, plus low molecular weight, mobile compounds (free fatty acids, diacids, and glycerol); and all of these substances are comparatively polar. The presence of hydroxy functional groups in long-chain fatty acids, for example, is known to increase significantly their solubility in polar solvents; and, by the same principle, fatty acid monoglycerides have greater solubility in polar solvents than diglycerides, which in turn have greater polar solubility than triglycerides (Schmid 1973). The increased ionic character of the residual polymer phase proposed in this scheme is particularly significant. Coordination between anionic groups on the polymer with multivalent metal ions, either at pigment surfaces or dissolved in the organic phase, is suggested as contributing significantly to cohesion of the paint film, increasing compactness and stiffness. Aging of paint films will, by both oxidation and/or hydrolysis, lead to increased polarity in the paint film and to the increasing anionic functionality of the paint binder. The formation of oxygenated functional groups (ethers, peroxy cross-links, hydroxyls, carbonyls, etc.) into both the stationary and mobile phases will inevitably increase sensitivity of the organic phase to polar and hydrogen-bonding solvents. Since metal ion—medium interactions would contribute strongly to the overall cohesion of the paint film, these liquids might also disrupt paint film cohesion and pigment binding by interfering with The mass of the organic phase of the paint film is known to diminish significantly as a consequence of aging, through loss of volatile scission products and molecules not immobilized in the polymer network. In combination with the increased cohesiveness conferred by internal electrostatic and H-bonding forces, this effect may result in severely aged paint films showing only low magnitudes of swelling in solvents compared to their young counterparts. It is conceivable that solvent sorption may cause the cohesion of old paint films to be interfered with by processes additional to, and more chemically complex than, the simple solvation swelling of the organic binder phase. Swelling—that is, gelation, softening and physical enlargement of the organic binder phase—may be a phenomenon of risk in cleaning that is most pertinent to relatively young or mediumrich oil paints. In the long run it may be that alternative investigative approaches will be necessary to examine properly the influence of solvents on the cohesion of paints that are appreciably aged. For the moment, however, there is value in reexamining the solvent-induced swelling behavior of typical young-mature oil paint films to clarify their sensitivities to solvents of various types. It is hoped that the results will help in providing a better understanding of paint-solvent interactions and will contribute to an improved theoretical framework for selecting solvents in the cleaning of paintings. Essentially, the goal is to provide a broader range of scientific evidence that will improve the precision with which practical conservators might deploy these chemical tools in execution of the craft of cleaning paintings. The primary objective has been to measure the degree and rate of swelling of selected paint films when immersed in a range of organic solvents repre-sentative of those commonly used in the cleaning of paintings and of specific chemical classes. |