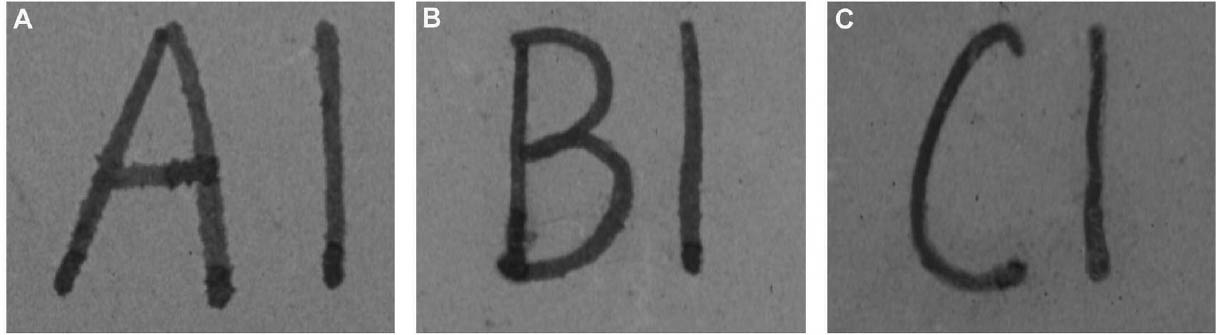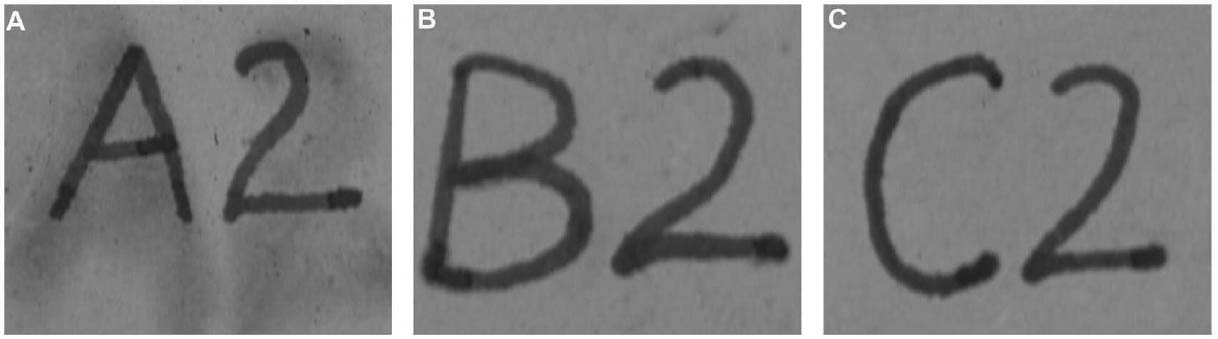THE USE OF CYCLODODECANE AS A TEMPORARY BARRIER FOR WATER-SENSITIVE INK ON ARCHAEOLOGICAL CERAMICS DURING DESALINATIONVANESSA MUROS, & JOHN HIRX
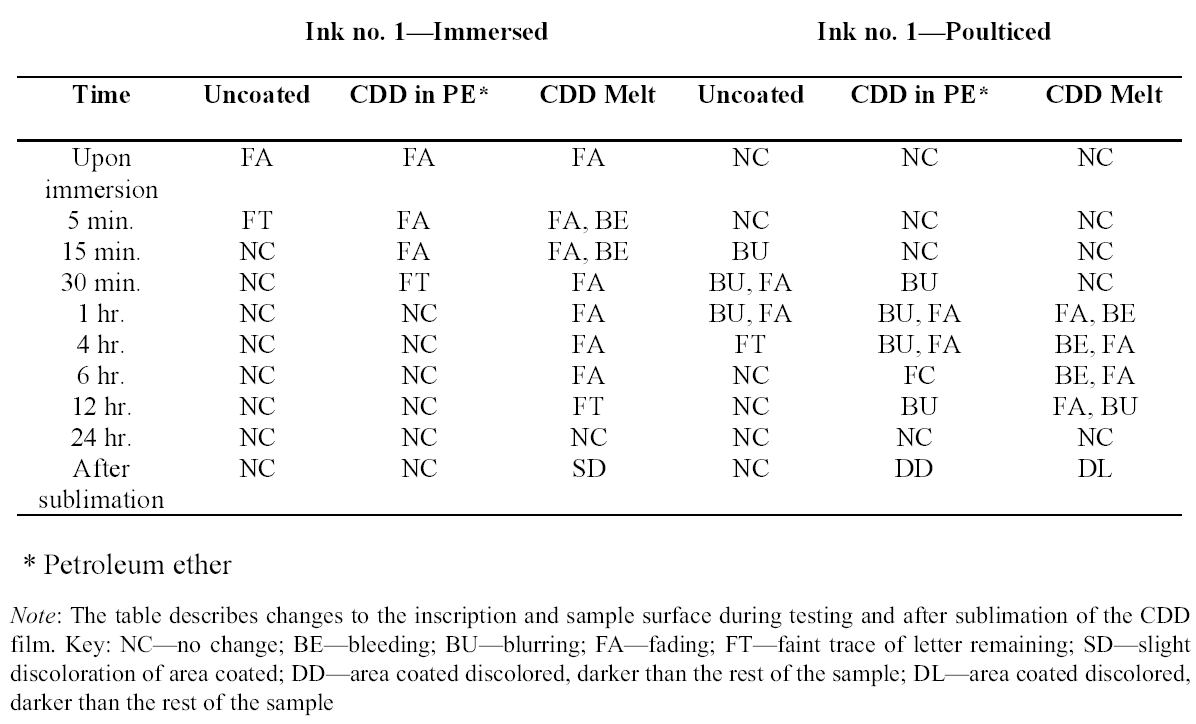
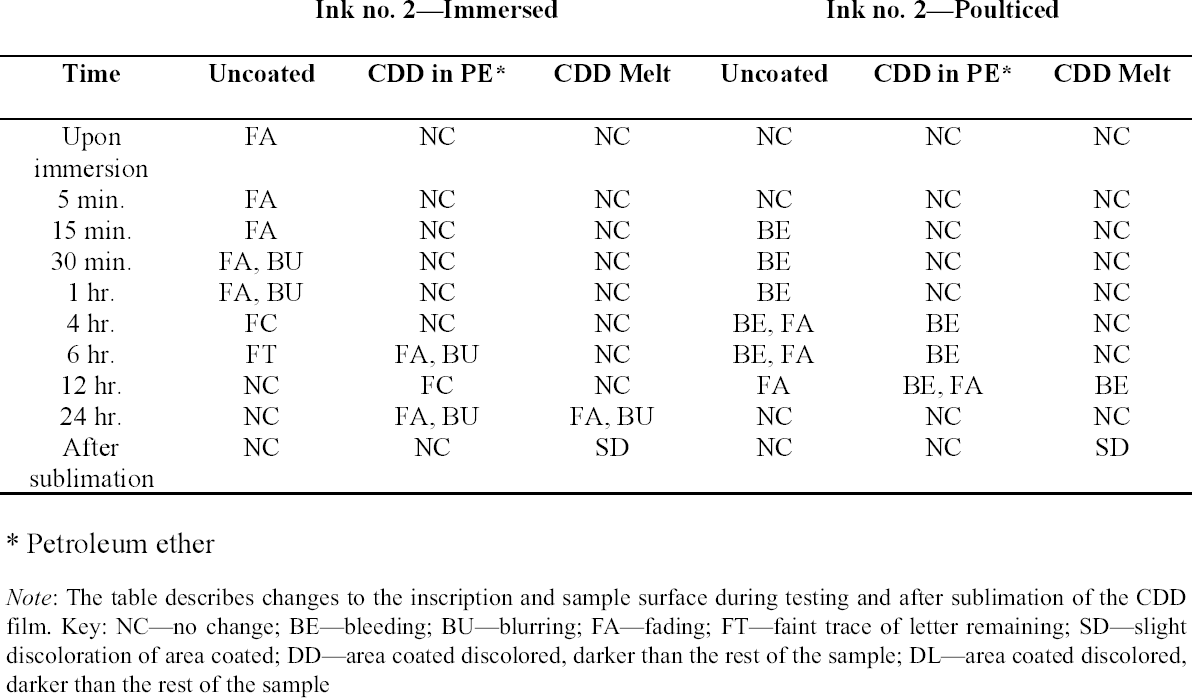
3 TESTS USING MODERN TERRACOTTA SAMPLES3.1 SAMPLE PREPARATION AND TESTINGBefore CDD could be used for the desalination of the Egyptian osctracon, the material was tested on inscribed modern terracotta samples, in a variation of tests conducted by Br�ckle et al. (1999) of ink on paper. These tests would help to determine whether CDD would be effective at protecting water-sensitive ink during desalination and what application method would produce the best barrier. Based on the results of the examinations into CDD's working properties and published successful treatments, the melt and CDD in petroleum ether (PE) (90% w/v) would be the application methods used for testing. Prior to coating, the terracotta samples were marked with ink of two varying water sensitivities. One set was marked with a Paper Mate nylon fiber point pen (ink no. 1), which seemed to be very sensitive to water. The other set was marked with Higgins Fountain Pen India nonwaterproof black ink (ink no. 2), which was less affected by water. In addition to their water sensitivity, the inks were also tested to see if they were sensitive to petroleum ether. Neither was affected by the application of the solvent. Each of the samples, measuring about 7 mm thick, was coated with a 0.05–1 mm film of CDD. None of the samples were warmed during application, and they were kept at room temperature during testing. An uncoated sample was included for each ink type to see the effect of the desalination techniques on the unprotected ink. Two different desalination techniques were used for testing to determine if either had any effect on the behavior of the barrier films and inks. One set of samples from each ink type was immersed in deionized water, and another set was poulticed, using cotton wool and deionized water, both for 24 hours. The poultice material was applied to the back of the sample to easily observe any changes in the inscription. The inscriptions were examined at various intervals within a 24-hour period, after desalination and after sublimation of the CDD from the surface. 3.2 RESULTSThe CDD films were unable to completely protect the inscriptions marked with ink no. 1 (nylon fiber point pen) from damage (figs. 3, 4). The ink, whether coated by the melt or by petroleum ether solution, bled and faded. The extent of the damage observed was dependent more on the method of desalination used (table 3). The ink on the coated, immersed samples had a greater tendency to fade, while the ink on the poulticed samples was more likely to bleed. Cyclododecane proved to be ineffective at protecting this particular ink. Cyclododecane, however, was very effective at protecting ink no. 2 (nonwaterproof India ink) during both poulticing and immersion (figs. 5, 6). While the inscription on the uncoated samples bled and faded, the
The results of these tests concur with the results of treatments using CDD on works of art on paper, where the melt was found to be a more effective barrier than the solutions during aqueous treatments (Bandow 1999; Br�ckle et al. 1999). Br�ckle et al. (1999) also found, as was the case with the two inks tested in this study, that CDD films differ in their effectiveness as a barrier according to the ink's degree of sensitivity to water. The films did not protect ink no. 1, despite the application method, because the ink seemed to be extremely sensitive to water. The CDD barriers better protected ink no. 2, which was less sensitive to water. These differences in the behavior of cyclododecane CDD on different types of inks suggest that some idea of a particular ink's sensitivity to water should be known prior to using CDD during any aqueous treatments. The water sensitivity of the ink on the ostracon will have to be determined prior to treatment to help reduce the risk of any damage to the inscription. Surface examination of the samples, after sublimation of CDD from the surface, revealed a slight discoloration on areas that had been coated with cyclododecane. The surfaces coated with the melt appeared lighter than the rest of the sample. Under microscopic examination (10–40x magnification), these areas appeared rougher and more porous than the areas that were not coated with cyclododecane. Samples coated with the PE solution had darkened
Since not all of the test samples exhibited these types of discoloration, other factors besides the application of CDD may be causing the surface changes. Because samples previously coated for earlier examinations, which were not soaked or poulticed, did not exhibit any surface changes, it is thought the discoloration or changes in surface texture may have been due to the dissolution of soluble material in the untreated areas of the terracotta samples during the mock desalination. Solvent residues or incomplete sublimation of CDD from the samples could also be factors in the discoloration. The cause and nature of the discoloration are areas that will need further investigation. |