THE USE OF CYCLODODECANE AS A TEMPORARY BARRIER FOR WATER-SENSITIVE INK ON ARCHAEOLOGICAL CERAMICS DURING DESALINATIONVANESSA MUROS, & JOHN HIRX
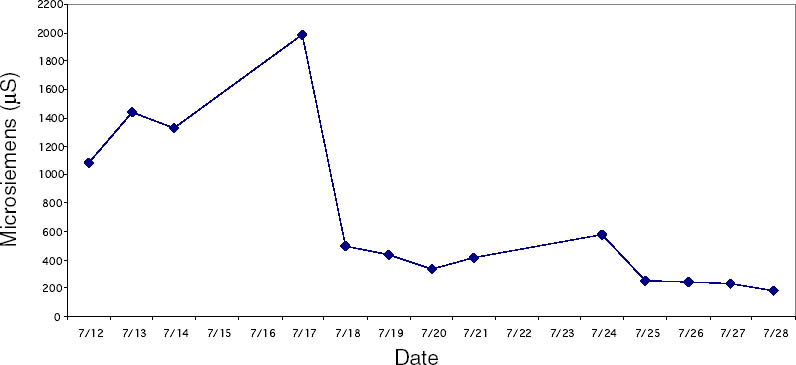
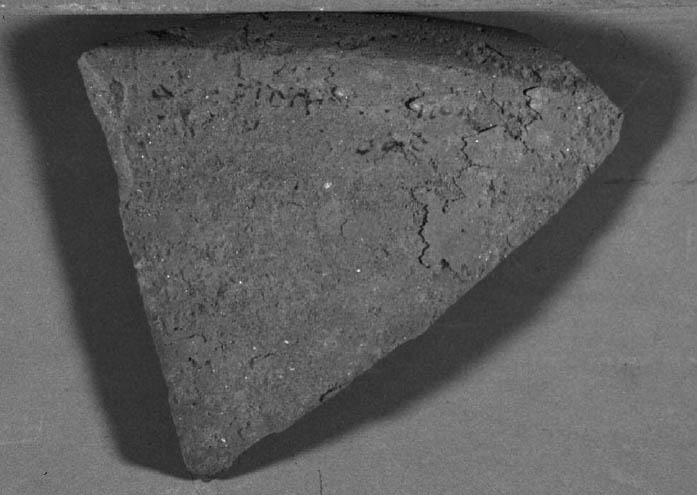
4 CASE STUDY: THE DESALINATION OF AN EGYPTIAN OSTRACONBased on the results of the tests on modern inscribed ceramic samples, CDD was used in the treatment of an ancient Egyptian ostracon that required desalination. The ostracon, in the collection of the Los Angeles County Museum of Art, suffered from a soluble salt problem. Having been previously stored in an environment with fluctuating temperature and humidity, the salts continued to crystallize and deliquesce within the pore structure, causing subsequent damage to the object. The surface of the ostracon was slightly flaky and powdery, with salt crystals visible. The salt damage was resulting in the loss of material, but, more important, in the loss of the inscription. A decision was made to desalinate the object, but because the ink or binder used was likely to be water sensitive, the inscription had to be protected first. CDD was chosen for the barrier because, in contrast Literature that discusses the writing of ancient Egypt (Forbes 1965; Singer et al. 1965) states that early inks were generally carbon black mixed with a binder, such as a gum or proteinaceous material, or in later periods an iron oxide–based material. In both cases, either the ink or the binder is considered water sensitive. A test was conducted to determine whether the ink, or binder, if present, was water sensitive. This test was also important because the age of the ink could cause it to behave differently than expected when in contact with water. To make these determinations, a small, disassociated flake with a small dot of ink was immersed in 30 ml of deionized water for 72 hours. The ink faded slightly upon immersion, but no other changes were observed. It was concluded that the ink was only slightly sensitive to water, and, therefore, based on earlier test results, the melt film should provide adequate protection for the inscription. A sample of the wash water used to observe the ink's sensitivity to water was used to determine what soluble salts were present. Microchemical tests were conducted to identify the presence of three common soluble salts in the wash water: silver nitrate to identify chlorides, ferrous sulfate for nitrates, and barium chloride for sulfates (Buys and Oakley 1993). The tests gave positive readings for the presence of nitrate and chloride salts. The CDD was melted on the surface of the ostracon using a Zephytronics Air Pencil ZT-2 (SMT Pin Point Soldering System), because it was felt the heated spatula, used for earlier tests, would apply too much pressure on the fragile surface. The air pencil was originally designed for soldering electronic and computer components, but it has been modified for use in conservation treatments, such as tape removal from paper (Stanley 1998). It melts or softens materials using warm air, where the air flow and temperature can be adjusted separately, as needed. The use of the air pencil would allow the cyclododecane to be melted using controllable warm air, without applying pressure to the object's surface. A low heat setting and the minimum airflow were used to melt the CDD on the ostracon. During application, pieces of solid cyclododecane were held just above the surface of the ostracon with tweezers. The air pencil was then held far enough away over the cyclododecane to allow the solid piece to melt, but not for the airflow to disturb any of the surface flakes. Only the area of the inscription was coated. The ostracon was desalinated by poulticing because immersion would result in the displacement of any flakes on the surface. Cotton wool wadding, dampened with deionized water, was placed behind the ostracon, where the surface was more cohesive. Poulticing from the back allowed the inscription to be visible during treatment so that any changes could be easily observed. The poultice material was changed every 24 hours, and the salt content monitored by soaking the poultice in 250 ml of deionized water and measuring the resulting conductivity. The ostracon was kept in a covered tray and sealed in a polyethylene bag during treatment to prevent the sublimation of the coating, something that occurred on some of the poulticed terracotta samples during testing. Desalination was completed after two months, when a series of similar conductivity readings were obtained within the acceptable level of 75–100 μS/cm (Buys and Oakley 1993) (fig. 7). The ostracon was left to dry out slowly in an open polyethylene bag, for about four to five weeks, until no cyclododecane remained on the surface and the inscription could be examined. Based on the sublimation rates from earlier tests, it is likely that some CDD remained within the ostracon and would require a few months to completely volatilize. Examination of the surface and inscription after treatment showed no bleeding or fading of the ink (fig. 8). The inscription appeared clearer after desalination. Comparison of the inscription before and after treatment showed no loss of the characters (fig. 9). No tidemarks were left, and no discoloration of
|