EVALUATION OF CLEANING METHODS FOR THE EXTERIOR BRICK AT THE BROOKLYN HISTORICAL SOCIETYCLAUDIA KAVENAGH, & GEORGE WHEELER
3 CLEANING3.1 GENERAL CONSIDERATIONSBased on the degree of soiling, it appears that the Brooklyn Historical Society building has never been cleaned. Heavy accumulations (described in the previous section) are present on both the brick and the terracotta. The soiling is aesthetically disfiguring in that it largely conceals the intricate beauty of this ornamented building (fig. 6, see page 72). The soiling also clogs the brick's pores and contains hydrocarbons that limit the penetration of water through the exterior faces of the brick. While this condition might have a protective effect similar to an applied water repellent, it also moves the drying front for the brick from its external surface to some point below the surface. Moving the drying front below the surface, in combination with gypsum-bearing mortars and absorptive brick, can promote damage to the brick (fig. 7). During rain events, calcium sulfate is drawn into the brick. In periods of drying, salts crystallize below the surface rather than appearing as efflorescences on the surface. Therefore, cleaning of the building may be considered both for aesthetic reasons and to increase the durability of the brick.
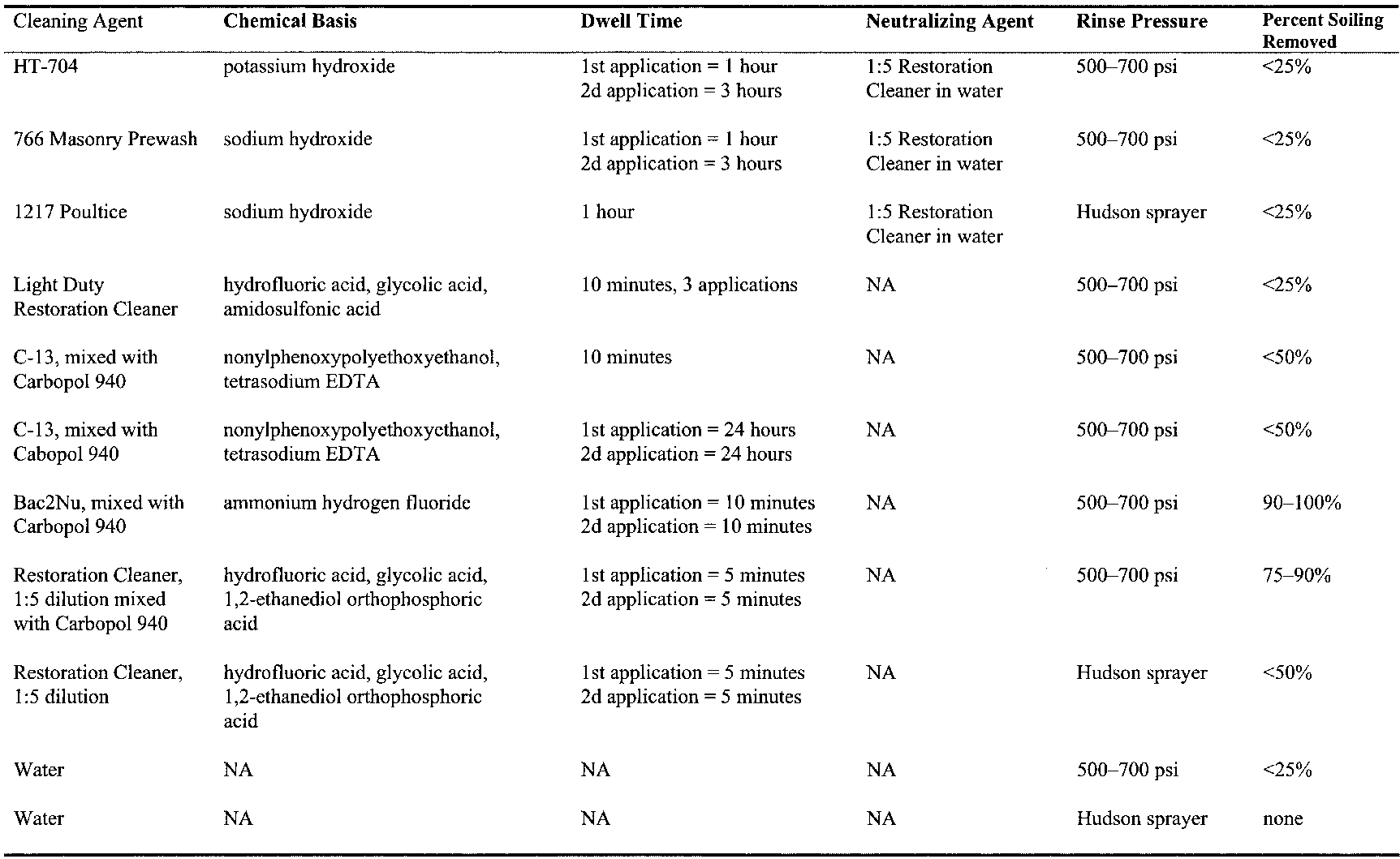
It was decided early on in the project to test only commercially available cleaning materials. This decision was due to the scale of the project and the difficulties often encountered in obtaining uniform compositions of nonproprietary cleaning materials when complex on-site mixing is required. With that premise in mind, cleaners that might be effective in removing soiling were examined. Use of water alone was discounted because water is not a primary factor in the removal of black soil from brick, as the soil tends to be tenaciously adhered to the silicates in the brick (Ashurst 1994). Cleaning methods for brick fall into two categories: abrasive and chemical. Due to the observed softness of the brick surface, abrasive methods were not tested. Commercially available chemical cleaners for brick include detergents, acids, and bases and acids applied in sequence (Ashurst 1994). Detergents, while not damaging to brick, are also generally ineffective in removing black soiling
All cleaning methods involve risk. Strong bases and hydrofluoric acid are known to dissolve silicates (Iler 1979; Ashurst and Dimes 1990; Moynehan et al. 1995). Their use in cleaning brick requires determining the dwell times and concentrations required to expeditiously remove the soiling with minimal effect on the substrate. Alkaline cleaners containing sodium hydroxide carry the additional risk of producing sodium sulfate salts if the cleaner is not fully removed from the brick (Ashurst 1994). Another consideration for cleaning this specific building is to minimize the mobilization of gypsum in the mortars and, to a lesser degree, in the brick. Considering the large volumes of water employed in many cleaning processes, the risk of drawing additional gypsum from the mortar into the brick is not an unreasonable concern. 3.2 CLEANING METHODSAn initial set of cleaning tests was performed on discrete areas of brick surface that could not be seen from any public vantage point. The initial tests were performed to obtain an understanding of what types of commercially available chemical formulations would remove the soil from the brick. Tests were performed on brick exhibiting a wide range of soiling conditions, from light to very heavy, and included detergents, acids, and bases and acids applied in sequence. Since the brick absorbs liquid so rapidly, chemical cleaners of low viscosity were formulated as gels, using either methyl cellulose or Carbopol 940 to keep them at the surface of the brick during the prescribed dwell time (table 1). (At
The typical procedure was as follows:
|