PERMANENCY OF REPROGRAPHIC IMAGES ON POLYESTER FILMHANNA SZCZEPANOWSKA, & WAYNE WILSON
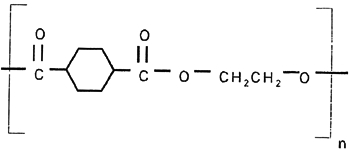
3 3. POLYESTER FILM AS SUPPORT FOR IMAGING3.1 3.1 GENERIC FILM STRUCTURE AND CHARACTERISTICSThere are approximately 220 polyester film manufacturing facilities around the world. The largest producers are DuPont, Mitsubishi, Toray, SKC, Teijin, Chiel, AGFA, Kodak, and 3M. Dimethyl terephthalate (DMT) and ethylene glycol are the primary chemicals used to produce commercial polyester film, for example, Mylar or Melinex (polyethylene terephthalate [PET]) (fig. 2). DMT can be replaced by terephthalic acid (TPA), which changes the production cycle slightly. DMT is used in a liquid form and TPA in a solid form. The primary chemicals undergo a complex process, which includes the following steps: (1) production of the monomer by combining ethylene glycol and terephthalic acid (or DMT); (2) addition of slip additives; (3) polymerization and removal of “excess” ethylene glycol; (4) filtration; and (5) extrusion. The surface characteristics of the polyester film that affect its durability as a support for reprographic purposes and contribute to image quality and longevity are decided fairly early in the production cycle.
The first decisive step is the successful chemical reaction of the raw materials. The second crucial step is the introduction of the slip additives prior to polymerization. The third step is the polymerization of the monomer. During polymerization, some of the ethylene glycol is freed by the chemical reaction, and this “excess” ethylene glycol must be removed for polymerization to proceed. Once the polymer has reached the desired molecular weight, it is then extruded into a cast sheet. After extrusion, during stretching of the film, crystallization of the polymer begins and removal of “excess” ethylene glycol takes place. Crystal growth occurs in the heat-setting process (DuPont Films Enterprise 1996). Uncoated film is stable in ambient temperatures. In many applications it does not change, even when exposed to a temperature of up to 150�C (302�F).2 The film is inert to most organic solvents at ambient temperature but is hydrolyzed by strong acids and bases and, at elevated temperatures (above 100�C), by moisture (DuPont Mylar 1996). Mylar is resistant to attack by fungi and bacteria. Samples buried in soil for 36 months remained unchanged (DuPont Mylar 1995). The test report of the material by DuPont Polyester Films provided interesting information regarding the stability of polyester film. One study focused on the effect of sunlight and weather on the physical properties of Mylar polyester film, using both outdoor exposure tests and laboratory instrumental methods. In most applications, polyester film is presumed to have been embrittled when it retains less than 20% of its original tensile elongation. The test conditions for this study provided the maximum possible exposure of the film to ambient light and weather. While such conditions are not normally encountered in typical applications, they do help to establish the minimum performance one might expect. According to this report, polyester film did not indicate any degradation when used or stored indoors under ambient conditions over five years. Film used as book cover material was unchanged in mechanical strength after six years. However, there is no way to calculate accurately the indoor longevity of Mylar. The report also cites the effects of ultraviolet light on polyester film. The degradation is rapid where the sun reaches the film. The film surface is altered by the UV light, and the depth of the degraded layer increases with exposure time. For this reason, heavier, thicker gauges of film last longer. Film cracks when the exposed side is bent. 3.2 3.2 SURFACE COATINGSThe extreme chemical inertness of the polyester film base makes adhesion of a coating much more difficult than with paper or other porous surfaces. The film used in reprographic technologies is often coated on both sides. The image side may be coated with photoreactive materials, usually involving silver or diazo compounds, or with electrocharged coatings as used in Xerox processes. The most typical application processes for frosted and photosensitive coatings involve organic solvents. The purpose of the solvent is to swell the surface of the material to be sensitized (as in diazotype) so that the sensitizer can penetrate through part of its thickness (Kosar 1965). Most materials applied to polyester film are held to the film with polymeric binders. Diazo technology, for example, employs a vinyl acetate/crotonic acid copolymer (Kosar 1965) and butadiene acrylonitrile or butadiene styrene copolymer. Polyester film is pretreated early in the manufacturing process for use in reprographic technologies. Historically, the surface was mechanically “roughened” prior to chemical pretreatment to create a stronger bond between the coating and the film (Lubar 1996). Coating of the polyester film is necessary to create an “anchorage” for the embedded image. Successful imaging depends on combining the materials to ensure the correct chemistry among them—strong enough, yet sustaining physical flexing of the support without affecting the image. Each reprographic technology requires a specific type of coating, usually custom-developed in the reprographic plant. Consequently, it is nearly impossible to establish a correlation among the type of damage, type of coating, and reprographic technology. One of the most prevalent types of damage—discoloration—can be attributed to a range of chemicals that were used as a base for imaging and will be discussed later in this article. Products involving xerographic processes and application of toner require different methods of film-base preparation, such as application of a durable coating to bond to the toner. Requirements ensuring stability of the image in Xerox products are discussed in the next section. The application of various overcoats to polyester film, the stability of the imaging technique, and the anchorage of the coatings to the film all affect the durability of the image during use and storage. These features typically are controlled by the manufacturers, who convert polyester film into reprographic materials. Information on coatings, however, is proprietary, for it is the single element that most directly affects image adhesion to the polyester film and hence the commercial success of the supplier. |