PERMANENCY OF REPROGRAPHIC IMAGES ON POLYESTER FILMHANNA SZCZEPANOWSKA, & WAYNE WILSON
4 4. IMAGING TECHNOLOGIESThe literature on images and imaging covers a great variety of imaging techniques, but most of it applies to technologies on paper. Images have many applications today. Most imaging technology fits into one of five categories depending on the way the images are created: (1) electronic imaging; (2) photographic silver halide imaging; (3) electrophotographic imaging; (4) chemical imaging; and (5) graphic arts and mechanical technologies. The imaging systems identified in this study are in three of these categories: (1) dry silver (photographic silver halide imaging); (2) xerographic copies (electrophotographic imaging); and (3) diazo (chemical imaging). An evaluation of these imaging systems was based on the microscopic examination of coatings and images on the sample records. A brief description of each technique follows. 4.1 4.1 PHOTOGRAPHIC SILVER HALIDE IMAGING“Dry silver” is a term used commercially by 3M. Since Kodak-Asahi joined 3M, the product has been described as thermally processed silver materials (TPSM). TPSM, both paper and film, are materials that can be exposed to light and processed by the application of heat to obtain an image. The process, substantially dry, takes place in the temperature range 100–250�C (Encyclopedia of Photography 1984). It combines the photosensitivity and speed of silver halide photochemistry with a dry, thermal development derived from thermographic copying technologies. In current practice, the silver halide grains are suspended in a nonaqueous, nongelatin medium with silver developer. Upon exposure to light or electron beams, a latent image forms in the silver halide (Dessauer and Looney 1989). The product of this process is a hard copy of a silver halide photograph characterized by a sharp and clear image. Compared to other imaging processes, these images are the most stable. An example of the dry silver process of photography was found in samples of the DocuMaster system developed by Agfa Division of Bayer Corporation (Afga n.d.), as illustrated in figures 3a and 3b. The imaged film samples were donated for testing and have undergone the same sequence of experimentation as samples from original records. The samples showed no signs of damage or alteration after extended exposure to high levels of humidity. The coating and image were unaffected and were not detached from the film base. A small number of records examined were executed in a technique similar to DocuMaster, producing records that endured extended exposure to high humidity. The Standards Subcommittee IT9-4, which focuses on film stability, was established in 1988 with the purpose of preparing specifications for dry silver film. However, according to the most recent publication (Adelstein 1991), there is no activity in this field, nor is there any current indication of interest.
4.2 4.2 ELECTROPHOTOGRAPHIC IMAGING—XEROXXerography was invented by Chester Carlson (1901-1968) as a nearly real-time, dry-processed, inexpensive office copying system. It is an electrophotographic imaging system. The term “electrophotography” evolved in the 1950s to refer to systems that create images based on electron charges and transfer them onto paper or film. The positive-charge image formed on the photoconductor (toner image) is transferred to the paper or film support by a charging sequence and is thermally fused to the support to make the final image. Figure 4 illustrates the sequence of xerographic image production (Stolka 1989). Toner particles are composed mainly of thermoplastics containing 5–10% of colorant by weight (Scharfe et al. 1989). The function of the thermoplastic is to physically fuse and fix the image on the paper or film by heat, pressure, or a combination of both. Toners are usually fabricated by mixing pigment and polymer followed by melting. The need to employ high temperatures and light in the execution of the Xerox copy requires specific film and coating to withstand demanding conditions. In selecting developer material, a delicate and precise balance of physical, chemical, mechanical, and electrical properties is required, matched to the design characteristics of the machine in which they are used (Xerox Corp. 1992). If this balance is not achieved, the image does not hold to the substrate, as illustrated in figures 5a and 5b.
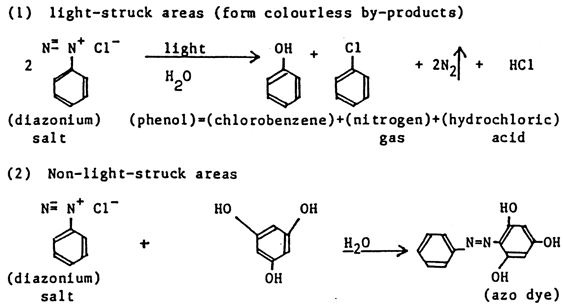
4.3 4.3 CHEMICAL IMAGING TECHNOLOGY—DIAZOMost record samples tested were found to be of the diazo imaging technology. The diazo process was first described by Green, Cross, and Bevan in Great Britain in 1890, but successful commercial products were not marketed until around 1923 (Dessauer and Looney 1989). The large-scale application of diazo printing in imaging technology dates to 1924. It has been used mostly for engineering drawings, color proofs, and microfilm duplicates. Diazo technology produces stable microfilms if one follows the recommended development steps (Kosar 1965). As a reprographic technique on paper, diazo is not a stable technique due to the developing process involved. Images on paper are water-sensitive and fade after prolonged exposure to air and light. The diazotype process gets its name from diazo compounds, which are light-sensitive substances. The diazotype is called “whiteprinting,” since a positive dye image of the original is formed on a white background. This process contrasts with blueprints, which provide a blue-colored negative copy from a positive master. In chemical imaging technologies such as diazotype, the image is created in coatings (applied to paper or film) by the action of light, usually ultraviolet light, or heat. The photochemical reaction takes place in a coating of diazo salts. A diazo print is created by superimposing the original transparent drawing on a sheet with a UV light-sensitive diazo coating, similar to the process of making contact prints in standard photography. The latent image is developed by exposure to ammonia and water vapor. The image is a product of diazo photolysis either in the dry state or in the gaseous phase. The support, either paper or polyester film, is coated with a photosensitive layer containing an aromatic diazonium salt and azo-coupling components. It is exposed to ultraviolet light or blue light. The photochemical sensitivity of the diazo compounds generally used in diazo papers is limited to a narrow spectral region, 375–420 μm. In the irradiated areas, the diazonium ion decomposes by loss of the diazonium group left in the shaded areas and reacts with azo-coupling components, forming azo dyes. Thus a positive image is formed (Kosar 1965; Laurencic 1987). The light to which the diazotype material is exposed must supply sufficient energy to destroy all the diazo compounds in the nonimage areas. The diazonium compounds used in diazotype coatings must meet certain requirements, including sufficient light sensitivity; solubility in water or a common solvent in order to be suitable for sensitizing solutions; and colorless photolysis products to ensure white background. The chemistry of the image formation in the diazo process is illustrated in figure 6.
Microscopically, a diazo image can be recognized by the smooth, clear surface of the photosensitive coating. It does not have the distinctive grain of silver particles found in the silver-based photographic technique. The azo dye images are essentially grainless (fig. 7). (For an example of the overall view of the record, see figure 9a.)
The diazo systems have the advantage of being inexpensive and easy to develop into the final image. The resolving power is about 10 times better than that of silver images. However, the light sensitivity of diazo systems is lower than that of silver photography by a factor of 10 to 100 (Zollinger 1994). According to the findings of standards organizations, diazo images are not considered suitable as “archival” media because they are dye-based. Specifications limits are given in ISO 8225 and IT9.5 for long- and medium-term films (Adelstein 1991). |