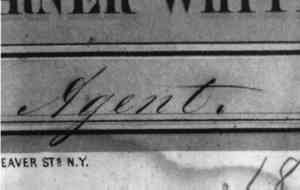CYCLODODECANE: TECHNICAL NOTE ON SOME USES IN PAPER AND OBJECTS CONSERVATIONIRENE BR�CKLE, JONATHAN THORNTON, KIMBERLY NICHOLS, & GERRI STRICKLER
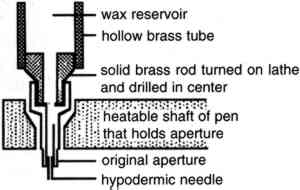
2 CYCLODODECANE AS A TEMPORARY FIXATIVE FOR WATER-SENSITIVE MEDIAThe aqueous treatment of paper has always been limited to some extent by the water-sensitivity of media associated with it. A number of treatment options have been developed that control moisture access to water-sensitive media during washing, among them blotter- or float-washing and use of a suction table. Only a few treatment choices allow the conservator to isolate or fix water-sensitive media for better protection during aqueous treatments. Several fixatives for the temporary isolation or impregnation of water-sensitive media have been used over the years. The most common is probably Paraloid B-72, although waxes and BEVA have also been employed (Rodgers [1988] 1994). In fine art paper conservation, the application of temporary fixatives is considered only with hesitancy. If used, they are commonly employed to protect during treatment small features of an object, such as an artist's signature or estate stamp. These features are often sensitive to aqueous treatment yet are difficult to test for sensitivity and allow for little risk-taking during treatment because, despite their small size, they often constitute a prominent 2.1 APPLICATION METHODA solid film of cyclododecane is formed either by cooling the molten material or by allowing an organic solvent to evaporate from a saturated solution of cyclododecane. The film conforms to the topography of the substrate to which it is applied (Riedl and Hilbert 1998). Its characteristics may vary depending on which of the two application methods is chosen (Hiby 1997), the porosity of the substrate, and the rate of film formation. In our tests, carried out at 45% RH, 21�C, a film formed after the evaporation of petroleum ether (boiling range 35–60�C) from a saturated cyclododecane solution brushed on an unheated glass slide was composed of large crystals, whereas cyclododecane applied in molten condition to a glass slide contained smaller crystals (fig. 1). Preliminary trials on a test object (“DRAFT” stamp, see sec. 2.2.1) showed that molten cyclododecane provided a better barrier than a solvent-based film for the protection of water-sensitive media. Hiby (1997) had melted cyclododecane using a heated spatula. To refine our treatment so that it would allow the isolation of narrow ink lines or other small-scale features on paper, we slightly modified an electric wax-melting pen (Kistka) that can be heated to 100�C. The wax reservoir of the pen was enlarged by a latheturned attachment fashioned from brass rod and brass tubing that were soldered together. This modification allowed longer working times between refills of cyclododecane. Because the apertures supplied with the instrument were too wide to allow a precise application of cyclododecane, the no. 2 aperture was fitted with a section of a 27 gauge, 1/2 in. long hypodermic
2.2 TREATMENTSCyclododecane was tested on a modern office stamp and three historical samples of iron gall ink. The modern office stamp underwent a selection of test treatments that served as a standard in this project. An expendable letter address written in iron gall ink underwent a mock washing treatment. Two artifacts that carried iron gall ink inscriptions were treated in case studies. The cyclododecane film was allowed to sublime before the objects were visually examined after treatment for the degree of bleeding observed. 2.2.1 “DRAFT” StampPaper and media: A modern, red-colored commercial office stamp that spells out the word “DRAFT” was stamped evenly on late-19th-century book papers. The brightly colored stamp pad ink was chosen as a test material because, being a molecularly dispersed colorant, it is very water soluble and could easily be read for signs of bleeding. The two types of paper were of similar rag fiber content, comparable thickness and smooth texture. One paper was sized, presumably with alum-rosin size. It tested positive for alum using the Aluminon test and repelled a water droplet placed on the paper surface. The other paper was unsized. Treatment procedure: Cyclododecane was applied with the wax-melting pen to the individual letters of one set of stamps, slightly overlapping the edges of each letter. The material impregnated the paper completely in the areas of application. The stamps underwent treatment directly after the application of cyclododecane. One other set of stamps was treated in unfixed condition, and another set was fixed with a 15% solution of B-72 (1:1 toluene:xylene). B-72 was brushed twice on the stamps, slightly overlapping the edges of the letters. The fixed area appeared visually well impregnated with B-72, judging by the transparency of the paper. Four different aqueous treatments were performed on the three sample sets (deionized water, pH 8.5 with calcium hydroxide): humidification, float washing, immersion washing, and blotter washing. Each treatment was allowed to continue for different lengths of time: 5, 15, 30, and 60 minutes. After treatment, the samples were allowed to air-dry on a screen. They were then visually compared with the untreated controls. Results: Cyclododecane compared favorably with B-72. Both materials effected substantial protection of the “DRAFT” stamp (table 1). With increasing duration of the treatment, however, both fixatives failed to protect the stamp pad ink completely from water penetration. The samples treated by immersion showed, as expected, the greatest degree of lateral bleeding of the ink. The unsized paper was more susceptible to the penetration of water than the sized paper, and the stamps on the unsized paper therefore suffered more damage than those on the sized paper. This difference was especially noticeable in the treatments that allowed greatest water contact with the samples, float washing and immersion. TABLE 1. DEGREE OF INK BLEEDING AFTER AQUEOUS TREATMENTS 2.2.2 Iron Gall Ink Letter AddressPaper and media: The address, dated 1848, was written on a sheet of handmade rag paper that had been folded into an envelope. The paper was thin and gelatin-alum-sized. It tested positive for gelatin using the Biuret test and positive for alum using the Aluminon test. The ink had slightly degraded the paper, causing local light brown and dark brown stains on the verso. The paper was otherwise in good condition and showed no signs of embrittlement in the areas of ink application. During water droplet testing, slight lateral migration of colored ink components occurred ca. 3 minutes after water was applied to the ink surface.
Treatment procedure: The letter address was impregnated with cyclododecane, which was allowed to extend slightly beyond the ink lines and penetrate the paper. The paper was float washed for 20 minutes in a water bath (pH 8.5
Results: Cyclododecane did not protect the ink inscription sufficiently, for several possible reasons. First, the thin, gelatin-sized paper expanded ca. 3% in the cross-grain direction when moistened. (The “DRAFT” stamp paper supports expanded only ca. 1% in the crossgrain direction.) A brittle cyclododecane film does not undergo the same expansion as the dampened paper sheet. During treatment, the fixative film may have developed fine crack patterns or openings invisible to the naked eye that would have allowed moisture to penetrate the fixed area. Further, the cyclododecane film inevitably was slightly flexed during the treatment procedure, a situation that may have caused cracks in the film, especially since the material was applied across a relatively wide area of the paper spanning the lengths of individual words (ca. 4 cm) (fig. 3). Third, the paper contained long fibers that extended from the unfixed into the fixed areas. Moisture was able to travel along or within these fibers and penetrate
2.2.3 Case StudiesChromolithograph: The advertisement was printed around 1865 on smooth, machinemade, uncoated bast fiber paper, most likely unsized or only weakly alum-rosin-sized. The paper absorbed water quickly and tested negative for alum using the Aluminon Test. The print was severely stained by tide lines and general discoloration. In a cartouche in the bottom center, the print was signed in iron gall ink, “E. C. Pasco, Agent.” The ink lines were crisp and dense along the vertical pen strokes. Although the ink did not show signs of sensitivity during a water droplet test (performed as in 2.2.2), the clarity of the inscription would have most certainly suffered during the extended aqueous treatment required to reduce the severe paper discoloration. Cyclododecane was applied before aqueous treatment. The ink lines showed no loss of crispness after ca. 80 minutes of float washing and immersion washing (fig. 5).
Chalk drawing: The drawing, made in 1912 in Palermo, Italy, had been fixed with a natural resin varnish (the varnish fluoresced yellowish under UV illumination). The machine-made drawing paper (watermark. “A. BINDA. E.C.T.”) was thick and slightly textured, and it may have been weakly sized with alum-rosin sizing. It tested slightly positive for alum using the Aluminon Test, tested negative for gelatin using the Biuret Test, and absorbed a water droplet only slowly. The drawing was signed in the lower margin by the artist in iron gall ink, “F. Balistrieri, 1912.” The signature did not show signs of sensitivity during a water droplet test (performed as in 2.2.2), but it was feared that its crisp appearance might be compromised during aqueous treatment. The objective of the treatment was to lighten the severely discolored blank paper margins. The application of cyclododecane to the signature allowed the paper margins to be covered by water during
2.3 RESULTSThe tests carried out in this brief study only partially replicated actual treatment situations. Under ordinary circumstances, the treatment of the highly water-sensitive “DRAFT” stamp would have not included such aggressive steps as, for example, immersion washing. Treatments of the stamp and the letter address showed that there are limitations to the efficacy of cyclododecane impregnation that must be considered when designing treatments using this material. Very water-sensitive media, especially if they are situated on unsized paper, may not be protected sufficiently by cyclododecane (and may, for that matter, be difficult to isolate with other types of fixatives). The treatment results of the chromolithograph and the chalk drawing suggest, however, that there are cases where media can be successfully protected by cyclododecane during aqueous treatment. Although neither of these iron gall inks showed any water sensitivity during pretreatment testing, it is very likely that they would have been affected if washed without the application of a protective coating. It seems, therefore, that cyclododecane may be suitable especially when media are suspected to show some, but not extreme, water sensitivity during aqueous treatment and when some measure of protection seems advisable. The effectiveness of cyclododecane as a fixative depends to some extent on the type and condition of the paper to which it is applied. Thick, dimensionally stable papers (such as the chalk drawing in 2.2.3) may be less likely to cause water penetration into the cyclododecane layer during treatment than thin papers and those that will expand greatly upon wetting (such as the handmade paper in 2.2.2). Papers made of strongly beaten fibers (such as the chromolithograph in 2.2.3) may be more successfully isolated with cyclododecane than papers that contain long, undamaged fibers (such as those in 2.2.2). Two measures may improve the impregnation of media with cyclododecane: applying the material from both sides of the paper and slightly warming the paper area during application. Cyclododecane appeared to sublime completely from the substrates after treatment. Selected samples were washed after the disappearance of cyclododecane to test whether the presence of the material had changed the wetting properties of the paper. None of the samples showed altered water absorption patterns in the areas where cyclododecane had been applied. Cyclododecane certainly cannot be regarded as a solution to all potential problems associated with the aqueous treatment of water-sensitive media on paper. The results of this short study indicate, however, that the material provides protection to media that can, under certain conditions, be equal to that offered by B-72. When compared to this and other fixatives, cyclododecane offers the following advantages:
|