POLARIZED LIGHT MICROSCOPY IN CONSERVATION: A PERSONAL PERSPECTIVEWALTER C. McCRONE
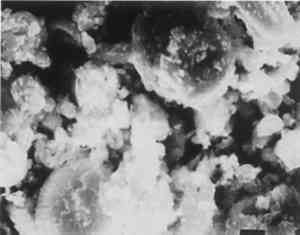
5 APPLICATIONSThe conservator-microscopist can study the identity, composition, or condition of objects in museum or other collections, including textiles, manuscripts, paper, leather, wood, metals, ceramics, stone, paintings—in short, any artistic or archaeological object. One significant application of PLM is for the characterization of a painting relative to the authenticity of its attribution to a particular time or painter. The pigments used in paintings all have a time of first use, although for some the date is shrouded in antiquity. Since about 1300, however, historical records and the finding of specific pigments in paintings of known date have fixed a list of dates of first use for most post-A.D. 1300 pigments (McCrone 1987). Some pigment compositions have multiple dates because they appear in different crystal forms (polymorphs) at different times or in different combinations. Titanium white (table 1) is an excellent example (McCrone 1990a). McCrone (1990a) also describes how PLM can identify rutile and anatase as such. TABLE 1 HISTORY OF USE OF TITANIUM WHITE PIGMENT Whiting and vermilion present similar situations. Whiting in the crystal form of rhombohedral calcite occurs in nature as the limestone mineral (fig. 17), as the skeletal remains of marine plankton (a coccolithophore) found in chalk (in the cliffs of Dover, for example) (fig. 18), and as a synthetic product dating only from the early 1800s (fig. 19). Vermilion also occurs in three forms: the mineral cinnabar, a dry process vermilion first produced by alchemists about A.D. 800, and a wet process form first synthesized as a pigment about 1700. I have observed the A.D. 800 vermilion in only a few medieval paintings, but notably in the Turin shroud blood-image areas (McCrone 1990b).
The condition of medieval pigments compared with their modern counterparts is also helpful in dating paintings. In general, pigments today are purer and more finely divided. Earth pigments, especially the iron earth pigments, tend to contain more quartz, limestone, and feldspar contaminants in earlier paintings. The synthetic equivalents of the earth pigments are, of course, free of extraneous mineral impurities. Lead white in the carbonate form, PbCO3, is hexagonal, as is the basic carbonate 2PbCO3�Pb(OH)2, but the latter is usually produced as flat plates, whereas the carbonate is elongated in the undc` axis direction. I have seen the latter in only three paintings: two paintings known to be by Edouard Manet and another painting also very likely a Manet (McCrone 1987). This situation invited a closer study of these elongated crystals of lead white in the hope of supporting, if not proving, the attribution to Manet. Two individual crystals each were isolated from the Infanta Margarita and Ballet Espagnol and checked for trace elements. Some of the data are shown in table 2. TABLE 2 COMPOSITION OF FOUR LEAD WHITE PIGMENT PARTICLES The Infanta data are from the likely Manet painting and the Ballet is from a known Manet painting in the Phillips Collection in Washington, D.C. The data for the first three crystals listed are very close. The fourth set of data differ especially for aluminum, magnesium, and silicon—common elements everywhere. I assume I did not clean that particular subnanogram crystal as well as I cleaned the other three; the largest was about 2 � 2 � 10 μm. In any case, the data in table 2 indicate that those crystals had a common history. I concluded that they could not have been more similar if they had been squeezed from the same tube of paint (McCrone 1987). My final conclusion was that the Infanta was very likely the long-lost Manet copy of a portion of a Vel�zquez painting in the Louvre; the conclusion of one “scholar” was that perhaps someone borrowed Manet's paints one day and produced the Infanta. This is another experience that convinced me not to spend any more time trying to authenticate works of art. Fortunately, there are many interesting problems for the conservator. For example, environmental effects can be evaluated and elucidated by microscopy. Louis Pomerantz once brought to me a painting by Fernand L�ger owned by a Chicago collector. The blue paint layers persisted in developing a white particulate efflorescence. A few crystals removed from the surface were recrystallized from a small drop of water on a microscope slide. I recognized the well-formed crystals immediately because they happened to be borax, a compound we use in many of our PLM courses as an example of a monoclinic crystal. It is a hygroscopic substance, dissolving in condensed moisture from your breath or even in high humidity atmospheres. The efflorescence that appeared on the blue paint surface was a result of moisture drawn from humid air migrating into the paint layer, which contained borax. The borax dissolved, followed by migration of the resulting borax solution to the paint surface, where it crystallized during dry periods. Katherine Kuh, then at the Art Institute in Chicago, suggested that L�ger, a potter as well as an artist, probably ran out of his favorite blue pigment and substituted a blue pottery glaze that contained borax as a flux. Another more serious environmental problem occurs in sulfuric acid–laden city atmospheres, polluted by the burning of fossil fuels. Sulfuric acid, in contact with paint layers that have acid-soluble and reactive pigments, such as zinc white, reacts to form sulfates. The crystals of zinc sulfate, identified by PLM, apparently result from zinc white reacting with sulfuric acid in city atmospheres. They are hydrated and thus occupy much more space than the original zinc oxide. The increased volume of the zinc sulfate formed during this reaction exapands and breaches the paint layer surface. If present in more than trace amounts, this expansion literally destroys that area of the painting. The expansion within the paint layer quickly breaks openings in the paint surface, facilitating an increasing rate of disintegration. Obviously, such conditions must be watched carefully, and, when they are first detected, heroic efforts must often be taken to prevent further damage. |