ULTRAVIOLET-FLUORESCENCE MICROSCOPY OF PAINT CROSS SECTIONS:JOHN M. MESSINGER
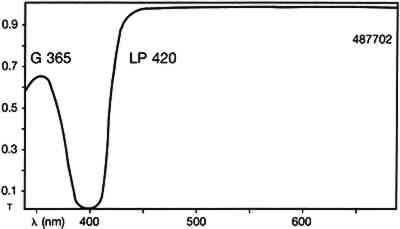
2 EXPERIMENTALThe various media were purchased commercially and painted onto glass microscope slides or After the known samples had cured, large representative chunks were removed from the test paintings and encased in cubes of Bio-Plastic polyester resin. After curing, the cross sections were ground and polished with odorless thinner as the cutting fluid. The samples were examined with a Zeiss Universal microscope with dark-field reflected light. The illumination was either in the visible light range with a 12 volt tungsten lamp or in the ultraviolet range with a high-pressure mercury vapor lamp. The filter set used with ultraviolet illumination was Zeiss catalog no 487702, which allows excitation of the sample with wavelengths up to 365nm and passes visible fluorescence above 420nm (fig. 1)
Photomicrographs were recorded on Kodak Ektachrome 200 daylight film (ED 135). When ultraviolet illumination was used, exposure times were four seconds. No filtration was used for ultraviolet illumination. To obtain proper color rendition with tungsten illumination, a Wratten 80A filter was required. DC-C7A is not commercially available, but its preparation from commercially available starting materials is easy. It was prepared, as outlined by Kinoshita, et al. (1975), by the following procedure: a 125 ml flask containing a magnetic spin bar was charged with 2.0 g of beta-cyclodextrin and 100 ml water. Fifteen minutes of stirring was required for complete dissolution of the cyclodextrin. Then 0.40 g of dansyl chloride was taken up in 3 ml anhydrous acetone and added to the cyclodextrin solution via dropper over 5 minutes. A fine yellow precipitate of the DC-C7A complex is formed in the flask during the course of addition. The flask and its contents were cooled in an ice bath for 30 minutes, centrifuged for 10 minutes, and the supernatant liquid decanted and discarded. The resultant lemon yellow powder was dried overnight over anhydrous CaSO4 at reduced pressure. The DC-C7A complex can be stored several months over CaSO4 in the dark. Just before use, about 10 mg of the DC-C7A complex is stirred into 5 ml of 8 M urea solution, and one drop of 10% triethanol amine in isopropanol (v/v) is added. The stain solution may be used immediately even though it takes 2–3 hours for all of the DC-C7A complex to dissolve. The solution remains effective as a stain for at least 4 hours. The stain was applied by soaking the sample cross sections in the solution for 8–10 minutes. The other fluorochromes were applied to the cross sections in solutions more dilute than those recommended by Wolbers and Landrey(1987), but otherwise the procedures were the same. It was felt that more dilute solutions would give The nonfluorochrome Sudan black (SB) was applied as recommended by Johnson and Packard (1971). A 60% ethanol:40% water (v/v) solution was saturated with SB. Cross sections were immersed in SB solution for about 30 minutes, then soaked in 40% ethanol:60% water (v/v) for 1 minute, then soaked in water 30 minutes. The amido black AB2 stain, as described by Martin (1977), was used exclusively: 100 mg of amido black was dissolved in 45 ml of glacial acetic acid to which is added 45 ml of 0.1 M aqueous sodium acetate followed by 10 ml of glycerine. Cross sections were immersed in the AB2 solution for approximately 5 minutes, rinsed with water, then soaked in 5% aqueous acetic acid for 5 minutes. |