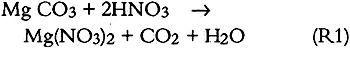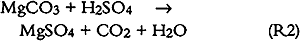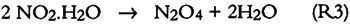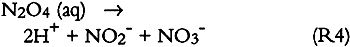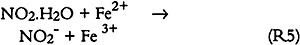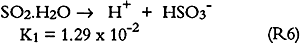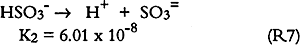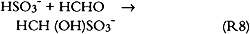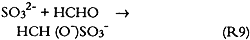EXPOSURE OF DEACIDIFIED AND UNTREATED PAPER TO AMBIENT LEVELS OF SULFUR DIOXIDE AND NITROGEN DIOXIDE: NATURE AND YIELDS OF REACTION PRODUCTSEDWIN L. WILLIAMS, & DANIEL GROSJEAN
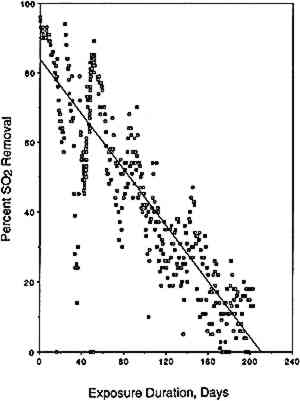
3 RESULTS AND DISCUSSION3.1 ABSORPTION OF SO2 AND NO2 BY PAPERThe amount of pollutant removed by the paper is given by the product of the air flow rate, the exposure duration, and the difference between pollutant inlet and outlet concentrations. The rate of pollutant removal is given by the ratio of the amount removed and the exposure duration. The amount of pollutant removed can be expressed on a relative basis, for example as a dimensionless fraction of the inlet polutant concentration:
This percentage, when plotted as a function of exposure duration, is indicative of changes an pollutant removal rate during the experiment,
Similar plots, not shown here but available elsewhere (Williams and Grosjean 1990), were obtained for NO2 removal in the NO2 exposure as well as for NO2 and SO2 removal in the exposure to a mixture of NO2 and SO2. The amount removed and the removal rates for SO2 and for NO2 were the same whether tested alone or as a mixture. Thus, not unexpectedly, uptake of SO2 and NO2 proceeded independently of each other's presence. Overall, the paper samples had a substantially larger capacity for uptake of SO2 than for uptake of NO2. For example, it took 40 days of exposure to NO2 (with or without SO2 present) for the paper samples to remove 10% of the NO2 and 200 days to remove the same fractional amount of SO2. This difference can be rationalized in terms of carbonate-pollutant interactions. It is well known that carbonate-coated substrates “collect” SO2 with high efficiency. In fact, several of the methods widely used to measure SO2 in ambient air involve sampling through carbonate-impregnated paper or glass fiber discs. Carbonate retains SO2 and other acids (hydrochloric acid, formic acid, acetic acid) with 100% efficiency but retains only a small fraction of the NO2(Okita and Ohta 1979; Grosjean et al. 1989; Grosjean 1990). 3.2 CAPACITY OF EXPOSED PAPER FOR ADDITIONAL POLLUTANT ABSORPTIONTwo observations suggest that the exposed paper still had capacity for more pollutant absorption. First, the paper samples were still absorbing 50% of the inlet SO2 concentration at the end of the 13-week exposure to the SO2 + NO2 mixture. Second, the initial carbonate content (estimated from gravimetric analysis) was larger than the amount of pollutant absorbed (calculated from inlet and exit concentrations and exposure duration). The initial carbonate content, in units of micromoles per
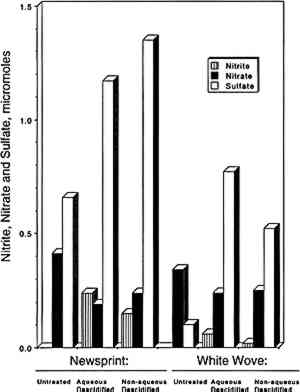
To further examine the paper capacity for additional absorption of NO2 and SO2, two exposures were repeated using much higher pollutant concentrations. Paper samples that had been exposed to 92 ppb of NO2 for 13 weeks were re-exposed to 1,200 ppb of NO2 for 4 days. During this second exposure, the paper samples removed 10–20% of the NO2. In the same way, paper samples that had been exposed to 100 ppb of the SO2-NO2 mixture were re-exposed to 1,000 ppb SO2 and 1,000 ppb NO2 for 7 days. Removal of SO2 was initially 80% and then gradually decreased to 20%; removal of NO2 was initially 65% and then decreased to 20%. These results clearly show that the paper samples could still absorb more NO2 and SO2 after 13 weeks of exposure to 100 ppb of NO2 or NO2 and SO2. 3.3 REACTION PRODUCTSThe reaction products identified included sulfate, nitrate, and nitrite, which were observed in all samples analyzed. Neither sulfite nor bisulfite was observed (detection limit = 6 μg per sample). Results for individual samples are not included due to space limitations and are available elsewhere (Williams and Grosjean 1990). Average sulfate, nitrate, and nitrite concentrations measured at the end of the expsoures are listed in table 1 and are summarized in figure 3. Several observations can be derived from these results:
TABLE 1 SULFATE, NITRATE, AND NITRITE CONTENT OF PAPER EXPOSED TO SULFUR DIOXIDE AND NITROGEN DIOXIDE
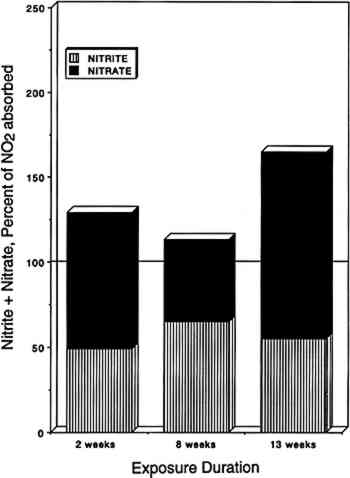
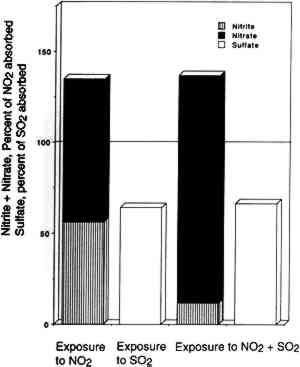
3.4 NITROGEN AND SULFUR MASS BALANCESCompared in table 2 are the amount of SO2 and NO2 absorbed (calculated from chamber inlet and exit concentrations, exposure duration, and chamber flow rate and corrected for the small amount lost to the chamber walls), and the amount of sulfate, nitrite, and nitrate measured in the paper samples by liquid chromatography. The same comparisons are illustrated in figure 4 for nitrate + nitrite vs. absorbed NO2 after 2, 8, and 13 weeks of exposure. Figure 5 compares TABLE 2 SULFUR AND NITROGEN MASS BALANCES FOR PAPER SAMPLES EXPLOSED TO SULFUR DIOXIDE AND NITROGEN DIOXIDE
Since only a small fraction of the total sample population, 2–5%, was withdrawn from the chamber at weekly or biweekly intervals, there is substantial scatter in the data summarized in table 2. Nevertheless, these comparisons indicate, that, on the average and within experimental uncertainty (see appendix), all of the absorbed NO2 could be accounted for as nitrate + nitrate, and sulfate accounted for 65 + 30% of the absorbed SO2. 3.5 NO2 ABSORPTIONUpon NO2 absorption on paper, nitrite may form by hydrolysis and by iron-catalyzed oxidation:
3.6 SO2 ABSORPTIONSulfur dioxide initially dissolves in aqueous media to form bisulfite and sulfite (S(IV)) as follows:
However, no S(IV) species could be detected in any of the sample extracts, thus suggesting that all bisulfite and/or sulfite initially present had been oxidized to sulfate either in the paper or during sample extraction and/or storage prior to analysis. There are many pathways for the oxidation of S(IV) to sulfate, S(VI). These include reaction with dissolved ozone, hydrogen peroxide, nitrite, or NO2; reaction with oxygen catalyzed by carbon; or oxidation catalyzed by transition metal ions such as iron, manganese, and copper. All pathways except those catalyzed by transition metal ions are unlikely to be important in our study since the oxidant To further investigate chemical reactions possibly taking place at the paper surface, several unextracted paper samples were analyzed by electron spectroscopy (ESCA) for sulfate, sulfite, bisulfite, sulfur, nitrate, nitrite, nitrogen, carbonate, carbon, oxygen, and trace metals (Williams and Grosjean 1990). One sample of each type was analyzed after exposure to the SO2 + NO2 mixture. With a detection limit equal to 0.1% of the total sample area scanned, no nitrite, nitrate, or carbonate could be detected. Deacidified newsprint contained sulfur, S(IV), in the form of sulfite or bisulfite (1%). Untreated newsprint contained oxidized sulfur, S(VI), that is, sulfate (1%). 3.7 TESTS FOR S(IV) OXIDATION DURING SAMPLE EXTRACTIONSince sulfite and bisulfite are easily oxidized to sulfate, they may actually be present after paper exposure to SO2 and may only be subsequently oxidized to sulfate during sample extraction. Thus, two experiments were carried out to test for the presence of sulfite or bisulfite in unextracted samples. In the first experiment, one-half of a paper sample exposed to SO2 for 29 weeks was extracted and was immediately analyzed for sulfate, while the other half was extracted with a 0.4% formaldehyde-water solution and then analyzed for sulfate. Any sulfite or bisulfite present would react with formaldehyde to form stable addition compounds:
Both samples were found to contain the same amount of sulfate, 116 � 16 μg, indicating that the original sample did not contain sulfite or bisulfite. In the second experiment, one-half of a paper sample exposed to SO2 + NO2 for 13 weeks was extracted and analyzed for sulfate. The other half of the sample was exposed to a flow of 1.2 ppm of gaseous formaldehyde in purified air for 16.5 hours (1600 μg of H2CO) prior to extraction. The unexposed and formal-dehyde-exposed samples contained 103 � 15 μg and 118 � 17 μg of sulfate, respectively. Again, if sulfite or bisulfite had been initially 3.8 BISULFITE STABILITY TESTAnother experiment was carried out to ascertain whether bisulfite could be oxidized on the paper sample or during extraction. One-half of a refrigerated control sample of aqueous-deacidified newsprint was extracted and analyzed for sulfate. The other half was spiked with 148 μg of bisulfite and allowed to dry prior to extraction and analysis. The control sample contained no detectable sulfate, as expected. The sample spiked with bisulfite contained 52 μg of sulfate, that is, 35% of the bisulfite was oxidized to sulfate prior to or during extraction. The spiked sample was reanalyzed after 20 hours. The sulfate content was the same, indicating that any bisulfite present in the extract was stable with respect to oxidation to sulfate for at least 20 hours after extraction. Results of the ESCA (electron spectroscopy) surface analysis, formaldehyde test, and bisulfite 3.9 TEST FOR OTHER SULFUR-CONTAINING REACTION PRODUCTSTo test for the presence of oxidizable sulfur compounds other than sulfite and bisulfite, a 5% hydrogen peroxide solution was added to an extracted, SO2-exposed sample. The sample treated with peroxide was re-analyzed for its sulfate content. No additional sulfate was found, thus indicating that no other oxidizable sulfur compounds were present. |