EXPOSURE OF DEACIDIFIED AND UNTREATED PAPER TO AMBIENT LEVELS OF SULFUR DIOXIDE AND NITROGEN DIOXIDE: NATURE AND YIELDS OF REACTION PRODUCTS
EDWIN L. WILLIAMS, & DANIEL GROSJEAN
APPENDIX
1 APPENDIX
1.1 MEASUREMENT UNCERTAINTIES
Uncertainties in nitrite, nitrate, and sulfate concentrations were calculated by propagation of errors. Linear calibration plots of peak height vs. μg of analyte injected were prepared each day samples were analyzed. The slopes and intercepts of the calibration plots were used to calculate the analyte concentration:
Fig. .
 |
where C is the analyte concentration in μg, P is the peak height in mm, S is the slope of the calibration plot in μg/mm, and I is the intercept of the calibration plot in μg. The uncertainty in the analyte concentration, σC, is given by:
Fig. .
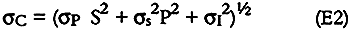 |
where σP is the uncertainty in the analyte peak height, σs is the uncertainty in the calibration slope (one standard deviation), and σI is the uncertainty in the calibration intercept (one standard deviation). The mean relative standard deviation (RSD) of the peak height from replicate injections was used to estimate σP. Mean RDSs were 3.3% for nitrite, 13.9% for nitrate, and 10.1% for sulfate (10, 17, and 17 sets of replicates, respectively).
Refrigerated controls and extraction solvent blanks contained some sulfate and nitrate but no nitrite. Thus, for nitrite, σC could be estimated directly from equation E2 and ranged from 1 to 8 μg per sample (equivalent to 5–19% for samples containing more than 10 μg nitrite). For nitrate and sulfate, concentrations in the paper samples were calculated by subtracting the nitrate and sulfate concentrations of the refrigerated controls and solvent blanks from the measured concentrations as follows:
Fig. .
 |
where X is the corrected analyte concentration in μg, A is the analyte concentration in μg, R is the analyte concentration of the refrigerator controls; and B is the analyte concentration of the solvent blanks. The uncertainty in X, σx, is given by:
Fig. .
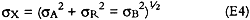 |
where σA, σR, and σB are the uncertainties in the analyte concentration of the paper sample, the refrigerated control, and the solvent blank, respectively. For nitrate, uncertainties calculated using equation E4 ranged between 1 and 6 μg per sample (5–27% for samples containing more than 10 μg nitrate), with a mean of 3 μg. For sulfate, uncertainties ranged between 5 and 32 μg per sample (2–63% for samples containing more than 10 μg sulfate), with a mean of 16 μg.
REFERENCES
Atherton, J. B., F. L.Hudson, and J. A.Hockey. 1973. The effect of temperature, light and some transitional metal ions on the sorption of sulphur dioxide by paper. Journal of Applied Chem. Biotechnol23:407–14.
Bennett, B. G., J. G.Kretzschmar, G. G.Akland, and H. W.de Koning. 1985. Urban air pollution worldwide. Environmental Science and Technology19:298–304.
Bredereck, K., A.Haberditzl, and A.Bl�her. 1990. Paper deacidification in large workshops: Effectiveness and practicability. Restaurator11:165–78.
Daniel, F., F.Flieder, and F.Leclerc. 1990. The effects of pollution on deacidified paper. Restaurator11:179–207.
DeKoning, H. W., J. G.Kretzschmar, G. G.Akland, and B. G.Bennett. 1986. Air pollution in different cities around the world. Atmospheric Environment20:101–14.
Edwards, C. J., F. L.Hudson, and J. A.Hockey, 1968. Sorption of sulphur dioxide by paper. Journal of Applied Chemistry18:146–48.
Grosjean, D.1990. Liquid chromatography analysis of chloride and nitrate with “negative” ultraviolet detection: Ambient levels and relative abundance of gas phase inorganic and organic acids in southern California. Environmental Science and Technology24:77–81.
Grosjean, D., A.Van Neste, and S. S.Parmar. 1989. Analysis of atmospheric carboxylic acids using single column ion exclusion chromatography with ultraviolet detection. Journal of Liquid Chromatography12:3007–17.
Hisham, M. W. M., and D.Grosjean. 1991. Air pollution in southern California museums: Indoor and outdoor levels of nitrogen dioxide, peroxyacetyl nitrate, nitrogen dioxide and chlorinated hydrocarbons. Environmental Science and Technology25:857–62.
Hudson, F. L., R. L.Grant, and J. A.Hockey. 1964. The pick-up of sulphur dioxide by paper. Journal of Applied Chemistry14:444–47.
Jarrell, T. D., J. M.Hankins, and F. P.Veitch. 1936. Deterioration of books and record papers. USDA Technical Bulletin 541, Washington, D.C.:U.S. Department of Agriculture.
Langwell, W. H.1955. The permanence of paper, part 4. Technical Bulletin of Paper and Board Makers Association36(1):199–207.
Langwell, W. H.1959. The permanence of paper, part 6. Technical Bulletin of Paper and Board Makers Association40(1):105–107.
Lienardy, A., and P.Van Damme. 1990. Practical deacidification. Restaurator11:1–21.
McCleary, J. P.1981. Paper conservation in Spain. In Preservation of paper and textiles of historic and artistic value II, ed.J. C.Williams. Advances in Chemistry series no. 193. Washington, D.C.: American Chemical Society.
Mihram, D.1986. Paper deacidification: A bibliographic survey. Restaurator7:81–98.
Okita, T., and S.Ohta1979. Measurements of nitrogenous and other compounds in the atmosphere and in cloudwater: A study of the mechanism of formation of acid precipitation. In Nitrogenous Air Pollutants: Chemical and Biological Implications, ed.D.Grosjean. Ann Arbor, Mich.: Ann Arbor Sciences Publisher. 289–306.
Smith, R. D.1975. The deacidification of paper and books. American Libraries6(2):108–10.
WilliamsE. L., II, and D.Grosjean. 1990. Exposure of deacidified and untreated paper to ambient levels of SO2 and NO2: Final report to the Getty Coservation Institute. Ventura, Calif.: DGA, Inc.
AUTHOR INFORMATION
EDWIN L. WILLIAMS II holds a M.S. degree in physical chemistry from the University of California, Los Angeles, and is a research scientist with DGA, Inc. Address: DGA, Inc., 4526 Telephone Road, Suite 205, Ventura, Calif. 93003.
DANIEL GROSJEAN holds a docteur es sciences degree in physical organic chemistry from the University of Paris and has done postdoctoral research at the California Institute of Technology, Pasadena, California. He is president of DGA, Inc., a private environmental research company founded in 1983 and directs research studies in atmospheric chemistry, air pollution measurements, museum air quality, and art conservation. Address: DGA, Inc., 4526 Telephone Road, Suite 205, Ventura, Calif. 93003.
|