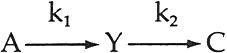DETERMINATION OF THE SPECIFIC RATE CONSTANT FOR THE LOSS OF A YELLOW INTERMEDIATE DURING THE FADING OF ALIZARIN LAKERobert L. Feller, Ruth M. Johnston-Feller, & Catherine Bailie
3 FORMATION OF A YELLOW INTERMEDIATEUPON CALCULATION OF THE CONCENTRATION of pigments necessary to match the spectrophotometric curves of the faded states of the test panels of alizarin lake and titanium white formulated in poly(vinylacetate), applied at complete hiding, the color-matching computations indicated that the addition of a yellow component was usually required. Figure 1 shows a typical result: along with the expected decrease in the amount of alizarin lake with increased exposure, there was also at first a rising, then a declining, concentration of a yellow component present in many of the exposed panels. Such behavior can be expected as the result of a sequence of chemical steps that may be represented by the equation
In our initial report,2 we demonstrated that the apparent rate of disappearance of the alizarin lake tended to follow equation ii. We further reported that the fading of alizarin and its yellow intermediate appeared to follow a sequence of events such as shown in equation iii and cited a specific first-order rate constant, k2, for the disappearance of the yellow. However, the method used to obtain the rate constant for this second step was not explained. We wish now to describe the technique used to obtain values of k2 by a simple graphical procedure. |