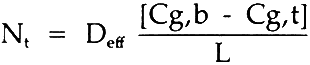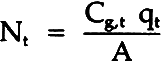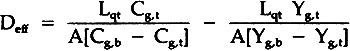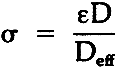THE DIFFUSION OF SULFUR DIOXIDE IN AIR THROUGH STACKED LAYERS OF PAPERMichael A. Dimitroff, & James W. Lacksonen
3 RESULTSThe determination of the effective diffusivity was made by coupling Fick's First Law with the sulfur dioxide material balance over the top chamber where sulfur dioxide was entering by diffusion through the paper block. Under steady-state conditions, Fick's First Law can be assumed as:
= molar flux of sulfur dioxide through the paper; Cg,b= molar concentration of sulfur dioxide, bottom; Cg,t= molar concentration of sulfur dioxide, top; L= diffusion length (block thickness); Deff= effective diffusivity. The sulfur dioxide material balance over the top chamber is:
= volumetric flow rate of gas out of top chamber A= area of diffusion Combining these equations gives:
TABLE 1 Effective Diffusivity Components of Sulfur Dioxide in Air Through Stacked Layers of Paper, Experimental Results For Diffusion Parallel to the Stacked Sheets of Paper Due to a Concentration Gradient in the Same Direction The tortuosity of the paper block is defined by the parallel pore model equation:10
= tortuosity ε= porosity D= binary gas phase diffusivity Deff= effective diffusivity in porous medium. Tortuosity represents the resistance to diffusion through a porous solid due to the influence of the pore structure. It assumes that a “tortuous path” delays the diffusion of a component compared to a “straight line” path. The concept of tortuosity is a |