THE DIFFUSION OF SULFUR DIOXIDE IN AIR THROUGH STACKED LAYERS OF PAPER
Michael A. Dimitroff, & James W. Lacksonen
4 CONCLUSIONS
Our research into the sorption/diffusion of gases into paper has concentrated on the steady-state evaluation of the effective diffusivity and tortuosity of sulfur dioxide in air. It can be argued that although we have thus far studied only one paper, the physical properties of the paper as prepared in a multilayered block should be reasonably similar to many other papers. With respect to the diffusion processes, it is significant to note that the diffusivity was about five times greater in the parallel direction compared to the normal direction. Of greater importance is the fact that the tortuosity in the parallel direction is 1.0 for this system. It's as if there were no resistance from the paper. This is equivalent to diffusion between parallel plates. Note also that the tortuosity calculation compares the effective diffusivity with the binary gas phase diffusivity. If the distance between the sheets were very small (less than one tenth the mean free path of the gas), than Knudsen diffusion would be expected. This apparently is not the case and the diffusion in the parallel direction is like diffusion through almost straight macropores. Since the paper blocks were prepared to simulate books, it is likely that diffusion into books via the parallel direction should be analogous to this study. Diffusion in the normal direction is more likely to be different and more dependent on the kind of paper, although the magnitude of this difference has yet to be determined.
It is suggested that suitable models for the diffusion of gases into books or documents which have a reasonable number of pages can be handled by assuming the pages are like parallel flat plates. Coupling the sorption phenomena to this model for the unsteady-state sorption diffusion process in one direction would require solving the differential equation (probably via computer)
Fig. .
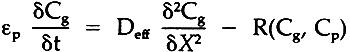 |
where:
εp= porosity of stacked paper Cg= concentration of gas X= distance into paper R= sorption rate equation, dependent on gas phase concentration Cg and absorbed phase concentration Cp
Preliminary sorption research has been done by the authors with the system described here. We have found that the sorption/desorption process is considerably more complex than the diffusion phenomena and future research should dedicate more effort to understanding this part of the penetration of gases into multilayers of paper.
|