THE DIFFUSION OF SULFUR DIOXIDE IN AIR THROUGH STACKED LAYERS OF PAPERMichael A. Dimitroff, & James W. Lacksonen
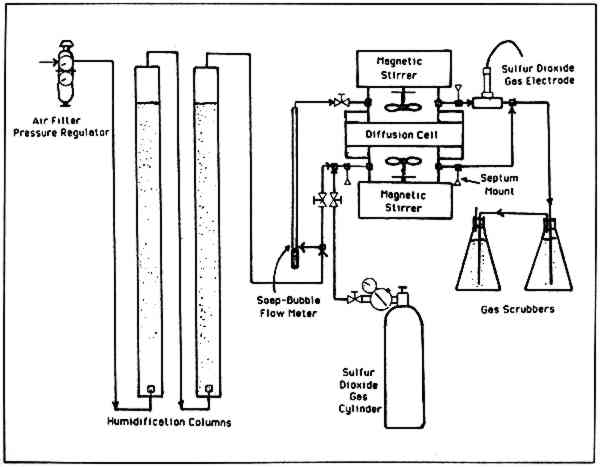
2 EXPERIMENTAL2.1 Analytic EquipmentA Sargent-Welch gas chromatograph model S-38585 was used for the gas analysis of sulfur dioxide in air. Accurate readings to 0.01% sulfur dioxide in air could be made with a 1 ml. sample. The thermoconductivity detector with helium carrier gas was used with a Supelpak-S column (a porous polymer packing of Porapak-Q type that is acid washed). Pure sulfur dioxide was used to prepare calibration samples with calibrated gas burettes. 2.2 The Diffusion CellA diffusion cell similar to that used to measure diffusivities in porous catalysts9 was constructed from 6 inch, schedule 80 PVC plastic pipe and glass. Two chambers were separated by a block of paper through which the gas of interest would diffuse. Each chamber was 310 cm3 in volume and was fitted with a plastic impeller which was magnetically driven to insure complete mixing. The paper block between the chambers was 10.0 cm by 10.0 cm (100 cm2 area) and was mounted in polyethylene 3.8 cm thick. Each chamber had � inch entrance and exit lines for admitting and removing gases. 2.3 The Paper BlockThe paper used in this study was 100% cotton fiber bond Crane's Kid Finish in Pearl White color. The cold extraction pH of the paper (TAPPI T428) was 5.1. Different orientations of the paper sheets were used in this study. For diffusion normal (perpendicular) to the stacked sheets, 226 sheets were stacked with the machine direction parallel, clamped between two pieces of Plexiglas, and silicone rubber cemented on the edges. There was no seepage of cement into the block. The block was mounted into the test section of polyethylene with silicone rubber cement. To avoid bulging, 11 cm. by 11 cm. sheets of similar paper were cemented to the top and bottom faces of the test section and weighted during drying. The block of paper gave an average of 59.22 sheets per cm of thickness. For diffusion parallel to the sheets of paper, similar construction methods were used. Five hundred ninety-two sheets 10.05 cm by 3.80 were cut with the width dimension being the machine direction. Since bulging was not a problem when this block of paper was mounted in the test 2.4 Experimental Setup and OperationThe experimental setup used to make the diffusivity measurements is shown in Figure 1. The air to the system was filtered and humidified by passing it through an aqueous solution of 83.6 wt % glycerine. Humidification measurements showed the air was 57% relative humidity at 73�F. Sulfur dioxide of 99.995% purity could be mixed with part of the inlet air stream to any desired composition. The top chamber of the diffusion cell had only humidified air entering, while the bottom chamber had humidified air with sulfur dioxide. The paper was “conditioned” in the cell for three days with humidified air and for an additional 36 hours with sulfur dioxide at the desired concentration to establish local equilibrium with the paper. (Previous separate absorption studies of sulfur dioxide with the paper had been done to insure that 36 hours gave no detectable change in absorbed sulfur dioxide.) After the paper was conditioned, it was considered to be at steady-state and 1.00 ml gas samples were taken
|