THE KINETICS OF FADING: OPAQUE PAINT FILMS PIGMENTED WITH ALIZARIN LAKE AND TITANIUM DIOXIDERuth Johnston-Feller, Robert L. Feller, Catherine W. Bailie, & Mary Curran
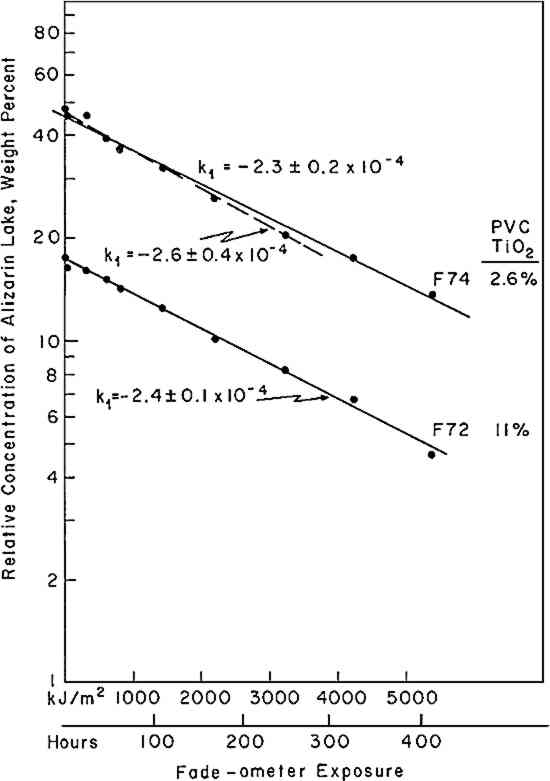
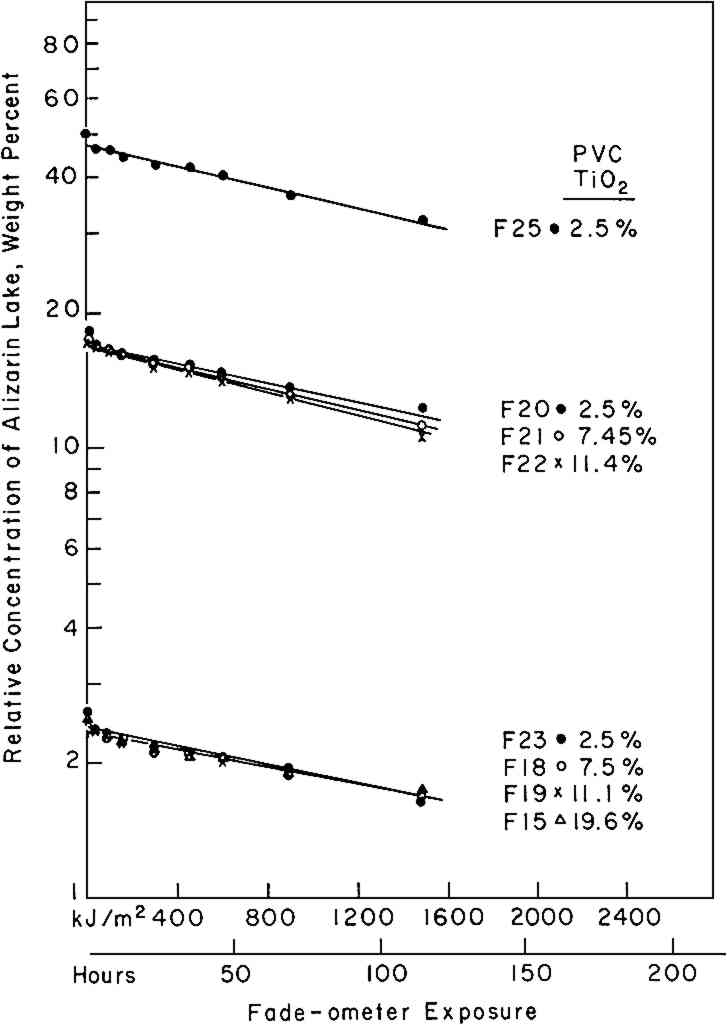
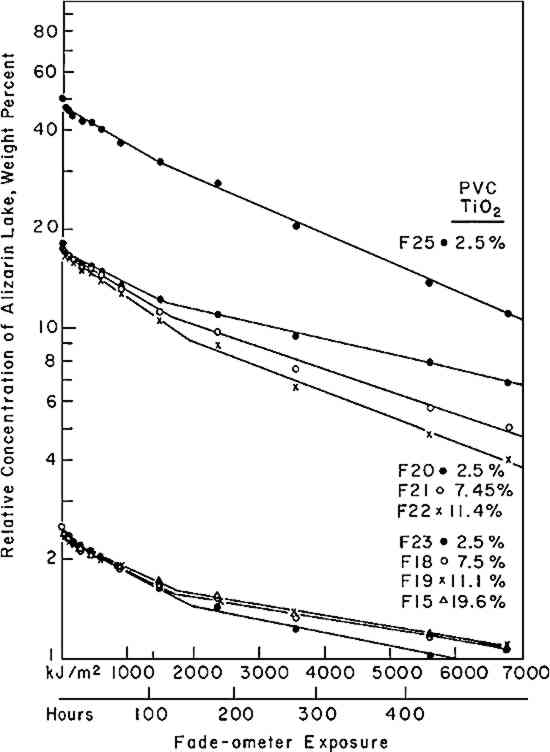
3 EXPERIMENTAL3.1 Pigments and VehicleThe vehicle used for these studies was a solvent-dry system based on a moderate molecular weight poly (vinyl acetate), Vinac� B-7 (Air Products Chemicals, Allentown, Pa.), a polymer of excellent photochemical stability similar to those sometimes used by conservators as a vehicle for retouching. The pigments used were alizarin lake (Ciba-Geigy X-686) and rutile titanium dioxide (du Pont Ti-Pure� R-960). The latter exhibits low photochemical activity.11 3.2 Preparation of the PanelsSeparate dispersions of the alizarin lake and of the titanium dioxide were made by ball-milling in a toluene solution of the poly(vinyl acetate) vehicle. These dispersions were then blended together to achieve the desired pigment and vehicle composition. The pigment ratios, alizarin lake/TiO2 on a weight basis, and the pigment volume concentrations (PVC) used in this study are described in Table I. Table I Compositions of Test Paints in Poly(vinyl acetate) The paints were applied to Eastman Kodak glass slides, 3�″ � 4″, by draw-down, using bars with a clearance of not greater than three mils. The draw-downs were made in a jig containing nine slides, of which only the center slide, uniform in appearance, was used. The glass was always weighed before coating so that the film thickness could be calculated from the weight and density of the solids in the paint. The purposes of applying the paint to glass slides were (a) to determine the completeness of hiding by measuring the reflectance over a white and over a black substrate and (b) to achieve a surface as smooth and free of irregularities as possible. The freshly coated panels were allowed to dry at ambient conditions for about an hour and were then baked overnight at 130�C to remove solvent. When necessary to achieve complete 3.3 MeasurementsSpectrophotometric curves of the paint panels initially and at intervals during exposure were measured using the integrating-sphere mode on a “Trilac” recording spectrophotometer (Leres, Alfortville, France) equipped with a small digital computer for 0% and 100% line corrections of the measured data at 10 nm intervals. Simultaneously, as the reflectance curves were being measured, computations at 10 nm intervals were performed to yield the CIE tristimulus values for Illuminant C, the chromaticity coordinates, and color differences all based on corrected reflectances. The CIE 1976 L∗a∗b∗ color-difference equation was used.12 Measurements were made with the specular (gloss) reflectance included (designated SCI or total reflectance, RT) and excluded (designated SCE) to determine changes in specular reflectance. Measurements were also made over a white and over a black substrate to make sure that the paint had remained at complete hiding throughout the exposure. Pressed barium sulfate was used as the white reference standard. 3.4 Exposure TechniqueThe panels were exposed in an Atlas Electric Devices (Chicago, Ill.) Model 65-WRC xenon-arc Fade-ometer� equipped with Pyrex�-glass filters which effectively eliminated radiation below 310 nm, closely simulating the range of spectral energy that passes through window glass. The air temperature in the chamber averaged 30.5� to 35�C; the black-panel temperature between 59� and 60�C. The ambient air in the chamber measured 20% R.H., with little deviation. Each time the panels were returned after measurement their position on the rotating rack in the Fade-ometer� was randomized with respect to the upper and lower positions. The painted glass panels were backed with a glass slide covered half with a stack of six white Millipore� filters (Millipore Corp., Bedford, Mass.) and half with black matte paint applied on aluminum foil. A pressed-wood support was arranged to leave an air space of ⅛″ between the samples and the black and white background. The purpose in mounting the samples over the black and white panels was to determine if there were any differences in the results owing to heating of the panels even though hiding in the visible spectral range was complete; none was found for these opaque samples. Because the intensity of the polychromatic irradiance in the Fade-ometer� declines with the age of the xenon-arc lamps, the rate calculations are expressed in terms of “exposure” rather than time—that is, the product of light intensity times clock hours. The Fade-ometer� is equipped with a light-monitoring device that measures the intensity of radiation on the samples at a selected wavelength, in this case 420 nm. Exposure, therefore, is expressed in kilojoules/meter2 (kJ/m2) as measured at 420 nm. During these experiments, 14.8 kJ/m2 was equivalent to about one hour of exposure. In the course of only a few hundred hours time, the distribution of the spectral power was not expected to change significantly. The fact that the rate constant determined in a second series of experiments at a later date (Figure 5) was identical within experimental error supports this expectation.
|