THE BLISTERING OF PAPER DURING HYDROGEN PEROXIDE BLEACHING
Daniel Clement
2 EXPERIMENTAL PROCEDURE
Seven degraded expendable nineteenth and early twentieth century lithographs were chosen as subjects for the experiment. These prints were cut into small pieces and the pieces evenly distributed into several groups which would each receive a different treatment. In this way, a number of treatments could be performed on parts of the same print and the variations in the amount of blistering and bleaching could be noted. Variations in bleaching procedures included changes in pH or concentration, variation in the kind of alkaline wash pretreatment, and the addition of ethanol or stabilizers to the bleaching bath. The amount of blistering and bleaching was measured after each treatment.
The expendable artifacts used are described briefly in Table 1. These prints were chosen because in a preliminary screening they were all shown to be susceptible to the formation of blisters during bleaching.7 Old prints were chosen in preference to other possible subjects, like sheets of artificially aged new paper, because it was believed that since these were examples of actual artwork, they would more closely represent some of the combinations of fiber furnish, sizings, coatings, staining and degradation that the conservator may encounter in practice.
Table 1. Description of the Papers Used
The purpose of the investigation was not to determine what kinds of paper were more likely to blister, but to see whether the problem of blistering could be abated by varying bleaching conditions.
The treatment procedures are described below and they are also outlined by the flow chart in Figure 1.
Fig. 1.
Flow chart for treatments.
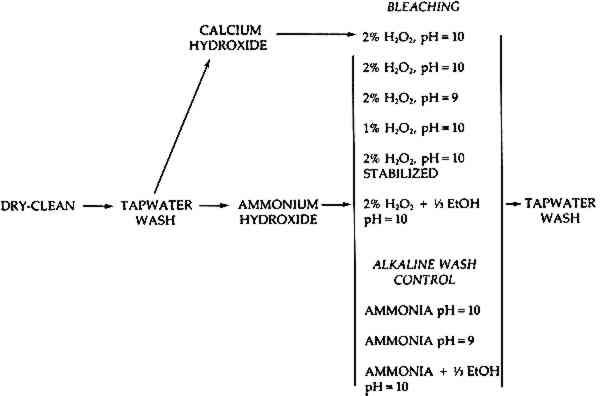 |
- All prints were drycleaned with Opaline eraser and then cut into 1 �″ � 3″ pieces and distributed into groups. The smallest sheet yielded 25 sections, while the largest provided 135. There were a total of nine treatment and control groups. While the larger prints provided enough pieces to be evenly distributed among all nine groups, the smaller prints yielded fewer pieces which were only used in certain of the treatment groups. Therefore, some of the experimental and control variations were performed using paper from all seven prints, while others were done using paper from only three or four prints. A total of 370 small sections of prints were used.Since it was observed in preliminary tests that the margins of papers were more prone to blistering than the rest of the sheets, note was made of the pieces which were taken from the edges of the prints and they were evenly distributed among the groups. These were later analyzed both as part of the entire group and separately.After the sheets were cut up and distributed into groups, each group was washed in tap water for � hour and allowed to air dry.
- All groups were given an alkaline wash for � hour at 25�C and allowed to air dry. (Reflectance measurements mentioned below in section F were taken here.) With eight of the nine groups this involved immersion in a bath of water brought to pH 10 with ammonium hydroxide, while the remaining group was bathed in a 50% saturated solution of calcium hydroxide. Margaret Hey has suggested that such a pretreatment may prevent damage to paper caused by hydrogen peroxide bleaching.8 The calcium hydroxide solution had a pH of about 12.9 Deionized water was used for all alkaline and bleaching baths.10
- Six of the nine groups were then each bleached in a particular hydrogen peroxide bleaching solution for � hour at 25�C All bleaching was done in non-metallic trays. For each treatment variation, all small pieces from a particular print were treated in the same tray, with 100 ml of solution being added for each piece. Fisher 30% hydrogen peroxide, stabilized with sodium stannate by the manufacturer, was the source of peroxide.The formulations of the various bleaching solutions are listed below.2% hydrogen peroxide brought to pH 10 with ammonium hydroxide. This was the standard bleaching solution to which the others were compared. The others each varied from this formulation in a particular way, for example, in pH or concentration.112% hydrogen peroxide in water brought to pH 9 (instead of 10) with ammonium hydroxide.1% hydrogen peroxide (instead of 2%) brought to pH 10 with ammonium hydroxide.2% hydrogen peroxide in water. A pH of 10 was maintained with sodium hydroxide, sodium carbonate and acetic acid. Magnesium sulfate and sodium silicate were added as stabilizers against decomposition.This formulation was suggested by Helen Burgess.12 While five different chemicals were added here, the pH and concentration remained the same as with the standard and it was decided to consider the experimental variable simply as the addition of stabilizers.A 33% volume equivalent of ethanol was added to a 2% solution of hydrogen peroxide in water.13 The pH was raised to 10 with ammonium hydroxide.
- The three remaining groups were alkaline wash controls. Since the total brightening resulting from an alkaline bleaching bath is presumably the combined effect of both bleaching and alkaline washing, alkaline baths were used as controls so that a clearer picture could be obtained of the brightening due to bleaching alone.The formulations of the alkaline wash controls are listed below. Each corresponds to one of the experimental bleaching solutions in pH and/or in the addition of ethanol.pH 10 ammonium hydroxide bath.pH 9 ammonium hydroxide bath.A 33% volume equivalent of ethanol is added to water and the pH raised to 10 with ammonium hyroxide.
- After bleaching, all papers were washed for five minutes in tap water and the blistering which occurred on each small piece was recorded with black and white raking light photographs taken on 35 mm film. The photography was performed while the paper was wet. The amount of blistering was later to be judged from these photographs. Figure 2 is a diagram of the box which was constructed in order to allow all of the photographs to be taken under precisely the same conditions of illumination and magnification, with the effects of ambient light eliminated. The blistering recorded on film was then compared to the kinds of blistering depicted on Figure 3A. This figure indicates how the amount of damage was quantified on a scale of 0 to 4. A rating of 0 meant that no blistering occurred at all, and 1 meant that very little blistering occurred. 2 was an intermediate rating described by either of the sketches in the third row. Severe blistering was given a rating of 3. This blistering might be evenly distributed over the surface, but it was also observed that the blistering often occurred preferentially over a limited part of the surface. A rating of 4 was used to describe the greatest amount of damage where the entire surface had blistered, or had blistered and delaminated. Although the categories may appear somewhat arbitrary, they seemed reasonable after observing the ways in which paper tended to be damaged by gas formation within the paper structure. One of the actual photographs taken using the setup described previously is reproduced for comparison in Figure 3B.
2
This box was constructed to make the raking light photographs used to record the blistering on the small bleached pieces of paper. The box was made of wood and painted flat black.
3A
Degrees of damage from blistering.
3B
Photograph of blistered paper taken using the photography box described in Fig. 2.In order to rate the blister damage to paper, contact prints were made of the 35 mm negatives and the photographic paper was cut ot the size of the individual frames. Three judges, all second-year Cooperstown paper conservation students, then grouped the photographs into the categories using the diagram as a guide. For purposes of analysis, the average of the three ratings, one from each judge, was used. Only with five photographs out of approximately 270 did the ratings of the three judges differ from one another by more than one unit. In these five cases, the judges were given the actual pieces of paper to evaluate, and the two-unit discrepancy was eliminated. It was considered acceptable, for example, if two judges gave a rating of 3 while the other gave a rating of 2. (The damage recorded by the photo in Figure 3B was given a rating of 3 by all judges.)
- After the five minute water rinse and photography, the papers were rinsed again for 55 minutes in tap water and allowed to air dry. The brightening of the paper was measured by taking total reflectance measurements of the surface of the dry paper at a wavelength of 416 nm both before the final bleaching step and again after the final tap water wash.14 The change in brightness was also measured for the alkaline wash controls, but no photographs were taken because no blistering occurred.
|
