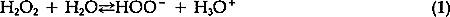THE BLISTERING OF PAPER DURING HYDROGEN PEROXIDE BLEACHINGDaniel Clement
1 INTRODUCTIONMANY PAPER CONSERVATORS USE hydrogen peroxide to bleach paper which has become discolored or stained. This bleach offers advantages over some of the other common bleaches. It decomposes into oxygen and water so that there is less chance that dangerous chemicals will be left in the paper; for example, it does not contain chlorine and so cannot leave chlorine residues. According to Burgess and Hanlan, hydrogen peroxide tends to degrade cellulose less than other common bleaches.1 While some conservators have noted problems of color reversion, Burgess reports results which indicate that paper bleached with hydrogen peroxide is less prone to color reversion than paper bleached with other common bleaches.2 Also, information presented in a TAPPI publication suggests that hydrogen peroxide improves the color stability of both bleached chemical paper pulps and bleached mechanical paper pulps.3 One problem that conservators sometimes encounter with aqueous hydrogen peroxide bleaching, however, is that blisters may form in the paper during bleaching. These blisters result when gas is produced and captured within the paper structure, causing small pockets. These gas-produced delaminations are visible because they stand in relief from the plane formed by the sheet of paper. While it is true that during actual treatments the bleaching can be terminated when the blistering starts to occur and the blisters can usually be brought back into plane as the paper dries, such mechanical disruption of the paper certainly is to be avoided. The active bleaching species4 associated with hydrogen peroxide is the perhydroxyl anion, HOO−. The reversible equilibrium which determines the concentration of this anion is described by
The irreversible decomposition of hydrogen peroxide, on the other hand, is described by
The present study reports results obtained in an experimental investigation into the extent of blistering during the bleaching of selected old papers when treated in a number of different hydrogen peroxide baths. The objective was to see which bleaching conditions and pretreatment procedures produced the least blistering for a given amount of bleaching. |