PENETRATION AND PLACEMENT OF ALKALINE COMPOUNDS IN SOLUTION-DEACIDIFIED PAPERGeorge B. Kelly, & Stanley Fowler
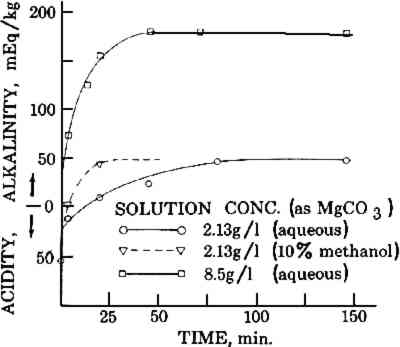
2 EXPERIMENTAL2.1 Rate of Penetration of Alkali During Immersion DeacidificationA solution of magnesium bicarbonate (solution A) was prepared by suspending 85 grams of magnesium carbonate light powder (laboratory grade, Fisher Scientific Co.) in 10 liters of distilled water, and bubbling carbon dioxide through the suspension until the magnesium carbonate dissolved (2 hours). The finished solution had only a slight haze. Solution B was prepared from solution A by diluting 1 volume with 3 volumes of distilled water and resaturating with carbon dioxide. Solution C was prepared from solution A by diluting 1 liter with a solution of 400 ml of methanol (certified A.C.S. grade, Fisher Scientific Co.) in 3 liters of distilled water and resaturating with carbon dioxide. Six sheets from an old book4 were immersed in solution A and removed at intervals of 4, 10, 25, 40, 80, and 140 minutes. The sheets were air dried and analyzed for alkali content by acidimetric titration.5 The process was repeated with solutions B and C. The results of the analyses were plotted against soaking time, as shown in Figure 1.
2.2 Migration During DryingThe migration of solids while drying was demonstrated with a stack of 8 sheets of facial quality tissues paper 10 cm square. These were assembled with sheets numbered 1 through 8 from the top down. The sheets were soaked separately in magnesium bicarbonate solutions at concentrations corresponding to 10, 5, and 2 grams of magnesium carbonate per liter of distilled water, dissolved by bubbling with carbon dioxide as in 2.1 (solutions D, E, and F, respectively). The sheets were then reassembled in numerical order on a glass plate while in the solution. The stack and plate were removed as a unit, and the excess water expelled by rolling with a rubber roller. This also compressed the stack into a composite sheet that would dry as a unit. The composites were then air dried on the glass plates. The individual sheets making up the composite were then separated and the alkali content of each determined by titration. Similar composites were frozen on a polished metal plate placed on the refrigerated shelf of a Vertis freeze dryer at −30 to −40�C, becoming completely frozen within 3 minutes of being composited. The composites were then dried at −40�C by evacuation to 5 microns pressure for 18 hours. The sheets were then separated and titrated for alkalinity.5 2.3 Modifying the Location of Alkali Within the SheetA stack of 8 sheets of facial quality tissues (10 cm square) was placed on the same size sheet of blotting paper saturated with a 10% solution of calcium chloride. The stack was then covered with a similar piece of blotting paper saturated with a 10% solution of ammonium carbonate and well rolled with a rubber roller to soak the stack of tissues. The stack was then separated, and the sheets were dried and washed separately for 30 minutes to remove unreacted solutions. After redrying, the alkali in each sheet was determined by acidimetric titration.5 The amount of alkali in the sheets was correlated with its position in the stack. 2.4 Effect of Solvent on Non-Aqueous DeacidifiersStrips of “as is” and oven-dried Whatman filter paper (#1), 25 � 80mm, were placed in a chromatographic jar with their ends immersed 8mm in 1% by weight solutions of magnesium methoxide and methylmagnesium carbonate in 1) pure methanol and 2) methanol-Freon TF, 1:5 by volume. The papers were removed at intervals of up to 15 minutes and inspected for the height of capillary rise of the solution. The position of the solvent line was marked. The papers were dried and then sprayed with a 0.1% solution of phenol red to locate the area containing alkali. The height of the alkali rise was measured and compared with the height of the solvent rise. Sheets of 26-mil blotting paper were painted on one side with 1% by weight solutions of magnesium methoxide and methylmagnesium carbonate in 1) pure methanol and 2) methanol-Freon TF, 1:5 by volume, applying sufficient solution to cause a readily perceptible dampness to the entire opposite side of the paper. After drying, the blotting papers were checked for surface pH on the front and back with a flat glass electrode pH meter. The front and back surfaces were also sprayed with a 0.1% solution of phenol red to visually check the location of alkali. The sheets were then split through the center and the exposed interior was sprayed with the phenol red to determine the depth of penetration of the alkali |