Comparison and Evaluation of Bleaching Procedures:
The Effect of Five Bleaching Methods on the Optical and Mechanical
Properties of New and Aged Cotton Linter Paper Before and After
Accelerated Aging
By Christa Hofmann, Dianne van der Reyden, and Mary Baker
I. Introduction
Bleaching is potentially one of the most harmful operations in paper conservation. Several studies have compared the effects of various chemical bleaches on the chemical and physical properties of paper 1,2,3 but few studies exist that compare the effect of chemical bleaching to aqueous light bleaching 3,5,6 before and after aging.
An earlier project at the Conservation Analytical Laboratory (CAL) on aqueous light bleaching conducted by Dianne van der Reyden 5 included the comparison of two hours of light bleaching to two hours of bleaching with either sodium borohydride (0.3%) or with hydrogen peroxide (3%), through the measurement of the color and tensile strength of naturally aged mixed pulp paper. Preliminary results indicate that the effects of light bleaching and sodium borohydride bleach appear to be comparable with respect to color and tensile strength before aging. Bleaching with hydrogen peroxide resulted in a significant decrease in both load (strength) and elongation to break. In the master's thesis project of Christa Hofmann4 at the Academy of Fine Arts in Vienna, foxing marks from biological origin on a collection of art nouveau prints were bleached. Seven bleaching methods were compared and the results subjectively ranked. It appeared that the conditions of the methods of application such as pre-washing, concentration of bleach, pH of the bleach bath, immersion time and post-rinsing highly influenced the results which were obtained. In order to investigate further these preliminary findings, a project was designed at CAL which expanded the previous work by:
pre-aging Whatman #1 filter paper (cotton seed hair/Enters, 98% alpha cellulose) for four weeks at 90°C and 50% RH in order to simulate a naturally aged paper for treatment;
comparing 5 different bleaching methods: hydrogen peroxide, sodium borohydride, aqueous light bleaching, calcium hypochlorite, potassium permanganate;
conducting accelerated aging of the samples after bleaching in order to simulate long-term effects of bleaching (under the same conditions as above);
evaluating and comparing the effect of each bleach before and after treatment by measuring pH and the following properties, before and after accelerated aging:
optical(measurement of color),
mechanical(measurement of tensile strength)
chemical (to be the focus of a subsequent project).
II. Experimental Design
In order to simulate new and "old" papers, the Whatman samples were divided, and half of the samples were artificially aged for four weeks (pre-aged) (Fig. 1). Samples of "old" and new papers were treated and then the samples were again divided. Half of the "old" and half of the new papers were subjected to artificial aging for another four weeks. This produced four groups of papers as seen in Figure 1. The treatment steps are summarized below and fully described in Appendix I. An overview of the bleaching methods is given in Table I.
Figure 1. Experimental Design
| Hydrogen peroxide A | sodium borohydride | Aqueous light bleaching | |
| Conc.: | 3% | 1% | |
| Time: | 30 min | 30 min | 2 hours |
| pH: | 8.9 | 10.45 | 9.3 |
| + conc. Ammonia | + Calcium Hydroxide. Light bleaching was carried out in the Weather-ometer in polystyrene bottles which cut off ultraviolet radiation at 360 nm; total irradiance was 6.9 kj /m2 | ||
| Hydrogen peroxide B | Calcium hypochlorite | Potassium permanganate A | |
| Conc.: | 3% | 0.05% | 0.1% |
| Time: | 30 min | 5 min | 30 sec. |
| pH: | 9.9 | 10.9 | 7 |
| + Sodium silicate. Stabilization according to Helen Burgess1. | + Calcium hydroxide | After bleaching the papers were immersed in a 1% potassium metabisulfite solution (pH 4.15) for 10 min to reduce the manganese ions. | |
| Potassium permanganate B | |||
| The same bleaching procedure as above was applied. The samples were left to dry overnight and immersed in the metabisulfite solution the next morning. |
GROUP I
a) Pre-treatment: The untreated Whatman 1 sample papers were washed in a saturated calcium carbonate solution, diluted 1: 1 with deionized water (pH 7.8), for one hour prior to bleaching. They were dried between blotters and felts under light weight pressure (c. 0.1 psi).
b) Bleaching: The various bleaching methods listed in Table I were carried out according to the standard methods for each different bleach as described in the literature 1,7. All solutions were prepared with deionized water.
c) Post-treatment: The washed controls and the bleached samples were rinsed for one hour in a saturated calcium carbonate solution diluted 1:1 with deionized water(pH 7.3). The water was changed after 30 min) and the paper was deacidified for 30 min in a calcium hydroxide solution(pH 9.1-9.4). The papers were dried between blotters and felts under light weight pressure (0.1 psi).
Controls: The washed control was subjected to the same pre- and post-treatment as the bleached samples. In addition an untreated Whatman 1 paper was included in the evaluation.
GROUP II
Half of the bleached samples, as well as the washed and the untreated control samples, were artificially aged for four weeks at 90 C and 50% RH in an Associated Environmental Systems HK-4116 Temperature/Humidity chamber.
GROUP III
Untreated Whatman 1 sample papers were pre-aged for four weeks at 90 C and 50 % RH. Pre-aging should allow a better comparison to the effect of bleaching on naturally aged paper.
a) Pre-treatment: as described above
b) Bleaching: as described above
c) Post-treatment: as described above
Controls: Untreated Whatman papers that were aged for four weeks served as untreated control samples. The pre-aged washed control samples were subjected to the same pre- and post-treatment as the bleached samples.
GROUP IV
Half of the pre-aged bleached samples, as well as the washed and untreated controls, were artificially aged for four weeks at 90 C and 50% RH.
III . Treatment Evaluation
The experimental procedures and the analytical instruments that were used are described in Appendix II.
a) Surface pH measurements
The surface pH was measured with a Corning Model 12 Research pH meter using an Orion model no. 81-35 flat surface combination electrode.
Despite the higher pH of some bleach solutions (for example sodium borohydride), the surface pH of the bleached samples remained similar; comparable to the washed control and more alkaline than the untreated control. Extensive rinsing and deacidification after bleaching is responsible for the uniform pH. Any differences caused by high pH of the bleach solutions disappeared after immersion in the alkaline rinse solution. Washing improves the pH of pre-aged papers and provides a better pH stability on aging compared to the untreated aged controls.
The pH is in the range of 5.7-6.3 for the bleached papers and of 5.7-6.2 for the pre-aged bleached papers. After aging there is a drop in pH which is approximately the same for all the samples. The pH in group II ranges from 5.4-5.6, in group IV (pre-aged) from 5.4-5.5. The pH of the washed control amounts to 6.3 and after aging to 5.6. The pH of the untreated control is 5.7 and after four weeks aging 5.3. The pre-aged washed control has a pH of 6.1 and after aging 5.5. The untreated control that was aged for eight weeks(equivalent to pre-aging plus post-aging) has a pH of 5.2.
b) Colorimetry measurements
The color was measured with a Hunterlab Ultrascan Spectracolorimeter using the CIE L*a*b* color notation system. Five measurements were taken from each sample and averaged.
As white Whatman paper was used for the experiments the differences in color before and after bleaching and before and after aging are not very pronounced. Pre-aging the Whatman paper resulted in only a slight discoloration due to the purity of the paper and the fact that it is unsized. Nevertheless certain tendencies in the effect of the bleaches, especially in group IV, could be observed.
L* value:
The L* value does not show any significant differences beyond the standard deviation due to the applied bleaches. The aged untreated controls, after four and eight weeks, were slightly darker than the washed controls and the bleached samples.
a* value:
There was no significant change in a* value caused by the different bleaching techniques as compared to the washed controls except for calcium hypochlorite which causes a pronounced drop in red for the preaged sample. For the preaged untreated controls, aging results in a significant increase in a* (an increase in redness) compared to the washed control and the bleached samples.
b* value:
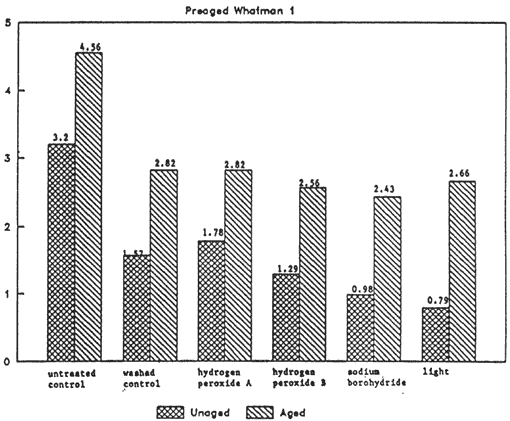
Figure 2(I). Colorimetry b* value (group I)
Figure 2(II). Colorimetry b* value (group II)
Figure 2(III). Colorimetry b* value (group III)
Figure 2(IV). Colorimetry b* value (group IV)
The greatest changes as a result of washing and bleaching can be observed in the b* value. The discoloration produced by aging is reduced by washing the paper. Washing also improves the color stability upon aging. The differences in color among bleaches and washed control are within a small range. It appears that the effect of washing and rinsing is the predominant factor in reducing the b* value of the Whatman paper. However, with respect to the b* value, the higher degree of yellowness after aging for potassium permanganate B could indicate degradation caused by the bleach: more carbonyl groups due to oxidation (perhaps due to residual magnesium oxide). The color stability after aging of the papers bleached with sodium borohydride is best. The lower b* value for sodium borohydride may be a result of the reduction of carbonyl groups, hence of the beneficial effect of this bleach. The stabilized hydrogen peroxide (B) shows slightly better color stability after aging than does the unstabilized hydrogen peroxide. The lower degree of yellowness for light bleached samples confirms the data of the previous work 8 (Fig. 2).
c) Tensile strength measurements
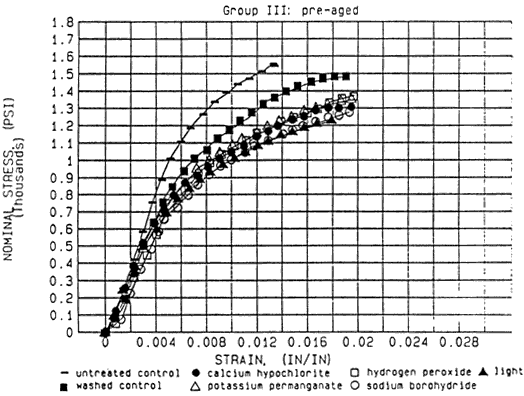
Fig. 3: Stress-strain curves, group I-IV. Hydrogen peroxide refers to hydrogen peroxide A, potassium permanganate to potassium permanganate A.
Figure 3(I). Comparative Bleaching Study
Figure 3(II). Comparative Bleaching Study
Figure 3(III). Comparative Bleaching Study
Figure 3(IV). Comparative Bleaching Study
The tensile tests were carried out with the Mecklenburg tensometer 5,15. Three specimens of each sample were measured. The averages are reproduced in Fig. 3.
Compared to the untreated controls, of the treated papers show a reduction in load to break and increased elongation in all four groups. Even after post-aging of the "old" treated samples, their elongation is longer than that of the untreated aged control (aged for the same amount of time)(Fig. 3). Aging of the untreated controls results in higher load (strength) and reduced elongation to break. The changes in stress and strain resulting from washing the sample paper exceed the changes brought about by bleaching the samples. Aging of the paper does not result in more significant differences between washed control and bleached samples.
Fig. 3: Differences in stress and strain compared to the washed control, group I-IV
Figure 4(I). Differences in stress (PSI) compared to the washed control
Figure 4(II). Differences in stress (PSI) compared to the washed control
Figure 4(III). Differences in strain (in/in) compared to the washed control
Figure 4(IV). Differences in strain (in/in) compared to the washed control
The differences among the bleaching procedures for stress and strain are relatively small. Bleaching with either sodium borohydride or light of the untreated Whatman results in a considerable increase in elongation which did not persist after accelerated aging. After eight weeks aging potassium permanganate and calcium hypochlorite have higher stress values than the washed control. Potassium permanganate caused a reduction in elongation as compared to the washed control. Sodium borohydride caused longer elongation than the washed control in all four groups, although the differences are sometimes very small (Fig. 4).
Low tensile strength and long elongation are sometimes considered positively in the paper industry, depending on the purpose of a specific paper type 9,11. Papers with a high rate of stress relaxation will more easily absorb a sudden increase in stress. A material that exhibits more viscoelastic behavior, a pronounced curvature and a high elongation to break may be considered tough 12. Toughness is defined as the area under the stress-strain curve. To focus attention only on the ultimate tensile load alone can lead to erroneous conclusions. A combination of lower ultimate stress and greater elongation can yield a superior paper for a specific purpose. Important properties of paper (softness, hardness, brittleness, pliability etc.) are governed by the ease of deformation of the sheet under comparatively mild stresses 10.
The stress-strain behavior might also give some indications about structural factors such as molecular weight, degree of crystallinity and degree of cross-linking. Hydrolysis can lead to an increase in the degree of crystallinity which could result in an increase in extensional stiffness of the paper and a decrease in its flexibility. Chain splitting in the amorphous region occurs in the early stages of hydrolysis of the cellulose fibers; this reduces chain entanglement, allowing an increase in crystallinity to take place 13.
The evaluation of the tensile data focuses on changes in strain. A longer elongation to break, as in the case of light and sodium borohydride bleaching (compared to the washed control), is considered positively. Less elongation and higher stress, as for potassium permanganate in group IV, are viewed as possible indications of paper degradation.
d) Scanning Electron Microscope (SEM) examination
For the SEM examination, a Jeol JXA-840 scanning electron microscope with a Tracor Northern TN 5502 energy dispersive analysis system was used. Samples, of cross-sections and of pulled edges after the tensile test, were taken from the washed controls and the bleached papers. The untreated controls were not examined by SEM.
Imaging
With regard to the observations with SEM, the greatest changes of the filter paper occurred after treatments with calcium hypochlorite and potassium permanganate, in the form of collapsed and shorter fibers. The papers bleached with sodium borohydride and light appear to be the least changed in visual appearance compared to the washed control. After aging, the differences between the washed control and bleached samples are smaller. The impact of the bleaches on the pre-aged papers is less obvious than that on the unaged papers.
Elemental analysis
After aging, dark spots appeared on the papers bleached by potassium permanganate method B. It was speculated that these spots might be manganese residues 14. However, energy dispersive x-ray analysis produced a high iron peak.
IV. Conclusions
Regarding pH, optical and mechanical properties, the differences between the untreated control and the washed control are greater than the differences between the washed control and the bleached papers. Washing seems to have a positive effect on the aged papers that were washed as well as on the washed papers that were aged. Washing and alkaline buffering appear to improve the pH, color stability and malleability of the paper used in this study upon aging.
The differences among the five bleaching procedures are within a relatively small range that is even smaller after aging. The differences may have been evened out by the washing and alkaline buffering steps. It appears that the impact of the conditions under which the bleaching procedure is carried out, like pre-washing and post-rinsing, pH, concentration and time of immersion, exceeds the effect of the different bleaches on the paper. This would confirm the findings of the previous empirical comparison 4.
Nevertheless, there are certain differences worth noting. Sodium borohydride exhibits the best color stability on aging; potassium permanganate B the worst. Papers bleached with sodium borohydride are the papers with the longest elongation after aging. Pre- and post-aged potassium permanganate and calcium hypochlorite, on the other hand, have higher stress and shorter elongation, which is similar to the behavior of the untreated control paper after aging. SEM imaging shows the most radical impact on samples bleached with potassium permanganate and calcium hypochlorite. Sodium borohydride and aqueous light bleached samples appear to be most similar to the washed control.
Chemical measurements including degree of polymerization (DP) and molecular weight distribution would be the next stage of this project. Unfortunately at this time it was not possible to undertake these measurements at CAL. Tensile strength measurements are mostly affected by the amount and quality of fiber bonding and the length of fibers9. Degradation caused by bleaching could occur in an intra fiber realm to an extent that is not advanced enough to have a considerable impact on tensile strength but is nevertheless significant. Measurements of chemical properties would be more sensitive to differences between washed control and bleached samples as well as among the various bleaching procedures.
Acknowledgements
The authors would like to extend thanks to the many other individuals who contributed to this project, most notably CAL staff, including Melanie Feather, Mary Ballard, Marion Mecklenburg, David Erhardt, Charlie Tumosa, Eleanor McMillan, Alan Postlethwaite and Lambertus van Zelst. The authors would like to acknowledge Verena Flamm and Gerhard Banik who supervised the master thesis work at the Austrian National Library. The CAL project was generously funded by the Austrian Foundation for the Promotion of Scientific Research.
References
1. H.D. Burgess: "Practical Considerations for Conservation Bleaching", J. IIC-GC, Vol. 13, 1988, pp. 11-26.
2. H.D. Burgess, J.F. Hanlan: "Degradation of Cellulose in Conservation Bleaching Treatments", J.IIC-GC, Vol.4, No.2, 1980, pp. 15-22.
3. F. Flieder, M. Leroy, J.C. Andreoli, F. Leclerc:"Comparative Study of Four Bleaching Methods", 1991, unpublished.
4. C.Hofmann, V.Flamm, G. Banik, R. Messner:"Bleaching of Foxing Stains on Art Nouveau Prints", preprints of the ICOM triennial meeting in Dresden, Los Angeles 1990, pp.472-477.
5. D. van der Reyden, M. Mecklenburg, M. Baker, M. Hamill:"Update on Current Research into Aqueous Light Bleaching at the Conservation Analytical Laboratory", The 1988 Book and Paper Group Annual, AIC, Washington DC 1988, pp 73-106.
6. A. Lienardy, P. van Damme:"Résultats de Recherches Expérimentales sur le Blanchiment du Papier", Studies in Conservation 34 (1989), pp 123-136.
7. H.D. Burgess, D. van der Reyden, K. Reyes: "Bleaching", Paper Conservation Catalog, AIC, Washington D.C. 1989.
8. T.T. Schaeffer, N.T. Baker, V.B. Hill, D. van der Reyden: "Effect of Aqueous Light Bleaching on the Subsequent Aging of Paper", JAIC, to be published.
9. H.G. Higgins, J. de Yong: "The Beating Process", in The Formation and Structure of Paper, F. Bolam editor, London 1962, Vol. II, pp 651-695.
10. W. Gallay:"Interdependance of Paper Properties", in The Formation and Structure of Paper, F. Bolam editor, London 1962, Vol. I, pp 491-532.
11. J. Casey, et al., Pulp and Paper: Chemistry and Chemical Technology. New York, 1980, Vol. III, 3rd. edition, pp 17791792.
12. D.F. Caulfield, D.E. Gunderson: "Paper Testing and Strength Characteristics", TAPPI Proceedings: 1988 Paper Preservation Symposium, Washington 1988, pp. 31-40.
13. E.L. Graminski:"The Stress-Strain Behavior of Accelerated and Naturally Aged Papers", TAPPI Journal, Vol.53, No.3, 1970, pp 406-410.
14. A.D. Baynes-Cope: "The Effect of Residues of Manganese Compounds in Paper on the Bleaching of Prints, etc.", The Paper Conservator, Vol. 2, 1977, p. 3.
15. D. Erhardt, D. van Endt, W. Hopwood: "The comparison of accelerated aging conditions through the analysis of extracts of artificially aged paper." AIC Book and Paper Preprints, 15th annual meeting, AIC, Washington D.C., pp. 43-55.
16. N.F. Mecklenburg: The role of water on the strength of polymers and adhesives. Doctoral dissertation, University of Maryland, College Park, MD.
Appendix I: Treatment Procedures
Pre-treatment: washing of samples
Calcium carbonate solution diluted 1:1 with deionized water, pH 7.8
The samples were placed on the screen (on the stainless metal frame) supported by polyester web. The web was sprayed with water. The samples were layered down and as well sprayed with water. Four samples were placed on the screen. Polyester web was put on top of the samples and another layer of four samples placed on it. In this way four layers with sixteen samples were prepared and immersed in the water bath. As many air bubbles between samples and polyester web as possible were removed. After one hour of washing the samples were transferred on the polyester web to a thick blotter. The Whatman papers were covered with polyester web and blotter. Excess moisture was blotted off. The top blotter and web were exchanged with dry blotter. The package was flipped around. The second blotter and web were as well exchanged with dry blotter. The blotter sandwich was put between felts and plexiglass under a weight of c. 0.1 psi. After 30 minutes the blotters were changed. On the next day the blotters were changed again. The samples remained under weight until they were treated (at least one week).
Bleaching Procedures and post-treatment:
Calcium Hypochlorite
Concentration: 0.05%
1 g calcium hypochlorite in 2000 ml deionized water
The pH is made alkaline with saturated calcium hydroxide solution.
Time: 5 min
pH: 10.9 before treatment
10.8 after treatment
The samples were placed on plexiglas and polyester web. On this support the pre-wetted paper(sprayed with deionized water) is immersed in the bleaching solution. After 5 min the sample is taken out of the solution, drained and immersed in a calcium carbonate solution (0.027% calcium carbonate solution diluted with deionized water 1:1), pH 7.3. The washing water is changed two times after 30 min. The test for chloride with silver nitrate was negative after the first water change. After washing the samples are immersed in a calcium hypochlorite solution (pH 9.1) for 30 min (1 ml saturated calcium hypochlorite solution is diluted with 1000 ml deionized water). Drying is carried out as described in the procedure for the pretreatment.
Potassium Permanganate: Method A
Concentration: 0.1% 2 g potassium permanganate in 2000 ml deionized water
Time: 30 sec
pH: 7
Concentration: 1%
20 g potassium metabisulfite in 2000 ml dei. water
Time: 10 min.
pH: 4.15
The samples were immersed in the potassium permanganate solution for 30 see, taken out, drained off and immersed in the potassium metabisulfite solution. They are washed in the calcium carbonate solution (pH 7.35) for one and a half hour, with a water change every 30 min. Deacidification in a calcium hydroxide solution (pH 9.1) for 30 min completes the treatment.
Potassium Permanganate: Method B
The samples are immersed in the potassium permanganate solution, taken out and drained off. On the polyester web they are transferred to a blotter and left to dry overnight. On the next day the papers are immersed in the potassium metabisulfite solution and then washed and deacidified as described above.
pH of calcium carbonate solution 8.5
Hydrogen Peroxide: Method A
Concentration: 3%
60 ml H2O2 in 2000 ml Deionized H2O
The pH is monitored by the addition of concentrated ammonia (1 ml ammonia to 2000 ml water).
Time: 30 min
pH: 8.9 before treatment
8.9 after treatment
Bleaching, washing and deacidification is carried out as described in the calcium hypochlorite treatment. pH of wash water: 7.8 (0.001% solution of calcium carbonate in deionized water, diluted 1:1 with deionized water) pH of calcium hydroxide solution: 9.2
Hydrogen Peroxide: Method B
Concentration: 3%
Stabilization according to Helen Burgess (J.IIC-CG, Vol.13, 1988)
Buffer solution: 24.4 g sodium carbonate in 100 ml deionized-water
20 g magnesium sulphate, 30 ml sodium silicate and 60 ml hydrogen peroxide are added to the buffer solution. A 2:1 solution is made up by adding 890 ml deionized water.
pH: 9.9 before treatment
pH: 9.8 after treatment
The stabilized solution was not decanted. The samples were immersed in the cloudy solution. Washing and deacidification were carried out as described above.
pH of wash water: 8.4
pH of calcium hydroxide solution: 8.9
Sodium Borohydride
Concentration: 1%
20 g sodium borohydride in 2000 ml all-water
pH: 10.45
Time: 30 min
Bleaching, washing and deacidification were carried out as described above. Due to the development of hydrogen gas the samples were pushed to the surface of the bleach bath. No blistering could be observed.
pH of wash water: 7.3
pH of calcium hydroxide solution: 9.3
Light Bleaching
Weather-ometer
420 nm filter
IR inner, IR outer filter
Total irradiance: 6.9 kilojoules/m2
Time: 2 hours
Filtration: ultraviolet radiation cut off at 360 nm by polystyrene bottles
The paper samples were mounted in polystyrene flat sided culture bottles (Falcon 3045). Each bottle was filled with approximately 600 ml calcium hydroxide solution(pH 9.3; 24°C). After 2 hours light exposure the pH dropped to 8.4 and the temperature rose to 33 C. Washing and deacidification were carried out after the light bleaching as described above. pH of wash water: 7.6 pH of calcium hydroxide sol.: 9.3
Washed Control
The control samples were washed in the calcium carbonate solution for one and a half hours. The water was changed after every 30 min. The samples were subsequently deacidified for 30 min.
pH of calcium carbonate solution: 7.1
pH of calcium hydroxide solution: 9.4
Appendix II: Experimental Procedures and Analytical Instruments
1. Artificial aging:
The paper samples were aged for four weeks in the dark at 90 C and 50% relative humidity in an Associated Environmental Systems HK4116 Temperature/Humidity chamber. These conditions have been chosen as suitable for artificial aging studies15. A set of Whatman 1 was sewn with cotton thread into plexiglas frames so that all four corners were anchored and the samples did not touch one another. However the samples did vibrate in the oven draft.
2. pH measurements:
The pH of all 36 samples was measured after treatment (except for the untreated controls), and of those samples that were accelerated aged after treatment(18 samples), after aging. A Corning Model 12 Research pH meter with an Orion model No. 81-35 flat surface combination electrode was used to measure the pH. The rinsed electrode, with a pendant drop of deionized water, was lowered onto a square paper sample(1.5 x 1.5 cm) on a polyethylene bag padded with blotters. The pH was recorded after 5 min. The electrode was calibrated with pH 7 and pH 4 buffer solutions before each measurement session.
3. Colorimetry:
The color of all 36 samples was recorded after treatment (except for the untreated control), and of those samples (18 samples) that were aged after treatment, after aging. The color was measured with the HunterLab Ultrascan Spectracolorimeter (D", 10° observer, 1/4 in 2 area of view) using the CIE L*a*b* color notation, where L* represents the degree of brightness (100 white, 0 black), a* the degree of redness (positive numbers) or the degree of greenness (negative numbers) and b* the degree of yellowness (positive numbers) or the degree of blueness (negative numbers). Five measurements were taken per sample and averaged.
4. SEM:
All samples except the untreated controls were examined with SEM (32 samples). SEM imaging and SEM analysis was carried out on a Jeol JXA - 840 A scanning electron microscope with a Tracor Northern TN 5502 energy dispersive analysis system. For imaging the samples were mounted on aluminum stubs and gold coated. For elemental analysis the samples were mounted on carbon stubs and carbon coated.
5. Tensile tests:
The tensile properties of all 36 samples were investigated using the Mecklenburg relaxation tensometer16 with a horizontal load applied in the machine direction to the paper strips. Narrow strips of uniform width were cut with a mat cutter. After measurement of the paper thickness in five places with a micrometer, the paper strips were mounted horizontally in the apparatus exposed to laboratory atmosphere. After an initial equilibration period at a gauge length an instructor in paper conservation for Johns Hopkins University's minute later the stress sustained by the paper strip was recorded. This process was repeated once per minute until the paper strip broke. Measurements were made on three strips of each paper. From these data, nominal stress (force applied per nominal crosssectional area of the strip) and strain (change in length divided by gauge length) were computed. Nominal stress was plotted as a function of strain for each paper strip.
Christa Hofman received her Master's Degree in Conservation from the Academy of Fine Arts, Vienna in 1989. She undertook internships at the Austrian National Library (1988-1989), Albertina Collection, Vienna (1989), and the Conservation Analytical Laboratory, Smithsonian Institution, Washington, DC (1990-1991). She has presented lectures and co-authored publications on topics including "Paper Bleaching" (ICOM Preprints, 1990), "Conservation of Tracing Papers" (ICOM Preprints, 1990), and "The Conservation of Costume Designs for Broadway Musicals, 1990-1925 (Restauro, 1989).
Dianne van der Reyden is Senior Paper Conservator at the Conservation Analytical Laboratory, Smithsonian Institution, Washington, DC. She has lectured and published on the "Technology and Treatment of a Folding Screen: Comparison of Oriental and Western Techniques" (IIC Preprints, '88), "Technology and Conservation Treatment of a 19th Century English 'Papier-Mache' Chair" (AIC Preprints '86), and "Technology and Treatment of a 19th Century American Time-Globe" (Paper Conservator, '86), as well as on coated and transparent papers (AIC BPG Annual, 89), and aqueous light bleaching of paper (JAIC '92, AIC BPG Annual '88). She also serves as an instructor in paper conservation for Johns Hopkins University's Material Science Program and CAL's Furniture Conservation Training Program. She is a Fellow of IIC and AIC, as well as a Past Officer of the AIC Board of Directors (1989-1991). She received her M.A. Art History and Certificate in Conservation from New York University in 1980, following internships at Harvard University Art Museums, the Library of Congress, and the Museum of Modern Art (NY).
Mary Baker received her B.S. in Chemistry in 1980 and her Ph.D. in 1986 in Materials Science with a specialty in Polymer Science from the Institute of Materials Science at the University of Connecticut. She has worked at CAL as a research chemist since 1987, collaborating with conservators on projects such as the effects of fumigation on materials; treatment and characterization of coated and transparent papers; light bleaching of paper; and methods development for analysis of micro-samples of paints, varnishes, and other materials. Her current research is on the modern polymeric materials in air and space artifacts and in modern paint media, their aging mechanisms, storage, treatment and display.
Christa HofmannPostgraduate Fellow
Dianne van der Reyden
Senior Paper Conservator
Mary Baker
Research Organic Chemist
Conservation Analytical Laboratory
Smithsonian Institution
Publication History
Received: Fall 1991
This paper supplements a poster presented at the AIC Annual Meeting in Albuquerque, 1991. It was submitted independently by the author, and was not delivered at the Book and Paper specialty group session of the AIC Annual Meeting. It has not received peer-review.