Update on Current Research Into Aqueous Light Bleaching at the Conservation Analytical Laboratory
by D. van der Reyden, M. Mecklenburg, M. Baker, and M. HamillThis paper was presented at the Book and Paper Specialty Session of the Annual Meeting of the American Institute for the Conservation of Art and Historic Artifacts, New Orleans, June 5, 1988.
1. Introduction
1.1 Conservation Literature (Treatment)
In 1980, in a ground-breaking publication on aqueous light bleaching of paper as a conservation treatment, Keiko Keyes noted significant reduction of stains in rag paper (without alum rosin size) immersed in a magnesium bicarbonate solution and exposed to sunlight, through a polyester film filter, for 2-4 hours [44]. Keyes observed empirically that, "Paper treated by this method of sun bleaching regains considerable physical strength, increasing its body and elasticity."1
In 1981, the effectiveness of sun bleaching on a wide variety of stain and paper types was confirmed by studies undertaken at the Center for Conservation and Technical Studies, Fogg Art Museum, and the first attempt to test physical changes in aqueously sun bleached papers, using the TAPPI Official Standard for Surface Strength of Paper (#T459 os-75), was published [59] 2. The visual effectiveness of light bleaching was particularly apparent in samples discolored by water stains, oil off-set, foxing, oxidation, matburn, and adhesive. 3 The results of the TAPPI surface strength test indicated that the critical wax number for bast and chemical wood pulp paper samples increased very slightly in aqueously light bleached samples, reflecting an increased resistance to picking (Fig. 1). Rag paper samples registered a slight decrease in critical wax number. All three paper types had alum rosin size.
Since 1981, paper conservators have published a great deal of information on aqueous light bleaching of paper [5, 6, 12, 22, 49, 56]. Many types of papers (including 17th and 18th century rag and 20th century alpha cellulose) have been exposed in a variety of buffered solutions (such as magnesium and calcium hydroxide and carbonates, magnesium citrate, calcium sulfate and ammonium hydroxide), to several light sources (ranging from sunlight to ultraviolet and fluorescent lights). While the visual effectiveness of the procedures continued to be confirmed, the understanding of changes in chemical, physical, and mechanical properties of paper, as measured by pH, colorimetry, viscosity, folding endurance, etc., remained incomplete. van der Reyden's findings in 1981 suggested a surface stiffening of some papers exposed to aqueous light bleaching; Branchick et al. in 1982 detected a drop in fold endurance of 18th century rag papers [12]; 4 and Savard in 1986 noted color reversion in artificially aged modern, mixed pulp papers [56]. 5
1.2 Scientific Literature (Testing)
Several factors complicate the evaluation of the long term effects of aqueous light bleaching on paper. Among these factors are: 1) the number of variables involved in the conservation treatment; 2) the lack of understanding of how these variables interact; 3) the limitations of some test methods used to detect and measure changes in paper and its components; 4) the problems inherent in interpreting the data; and 5) the lack of criteria to determine whether measured changes in paper, if significant, are ultimately beneficial or not. A survey of relevant scientific literature serves to shed some light on these factors.
1.2.1 Numerous scientific publications touch on some aspects of the variables inherent in the conservation treatment of aqueous light bleaching of paper. Many articles cover the effect on cellulose of photodegradation, water, temperature, alkaline degradation, swelling, and alum rosin sizing, among other things (for specific references, see the topic subdivisions under the Selected Bibliography). However, it is difficult to correlate the diverse scientific information, much of which is on dry paper, to aqueous light bleaching of paper as a conservation treatment. The treatment variables derive for the most part from a) the composition of the paper, b) the immersion solution, and c) the light source.
a) The composition of the paper comprises factors of furnish (fiber types, sizes, fillers, etc..), formation (chemical treatment, thickness, etc..), and degradation (products). Some relevant information on these areas is available. For instance, in respect to alum rosin size, Hon noted that it yellows rapidly in the light [42], which Kimberly asserts happens regardless of whether the rosin was previously bleached or not [45]. Kimberly further observed that rosin size will darken in enclosed carbon arc light (exposure time was 24-288 hours) and that light causes loss of rosin sizing. Launer noted that new rag paper is more affected by rosin than old rag and soda-sulfite paper [46]. Additionally, some of the degradation products, induced by ultraviolet radiation, and responsible for discoloration of cellulose, have been identified as the compounds xylose, D-glucose, D-arabinose, and cellobiose by Ranby [54]. Erhardt found that humid oven aging of Whatman filter paper #1 (composed of cotton seed hair fibers) produced xylose and glucose, although much less glucose was produced during dry oven aging [24]. 6
b) The immersion solution variables include immersion time, temperature, and alkalinity. Eldridge in 1982 noted the relationship between alkalinity and speed of aqueous light bleaching in her study? comparing calcium hydroxide to magnesium bicarbonate solutions [22]. 7 It remains to be seen whether or not this is related to increased swelling occurring in the water accessible amorphous hemicellulose regions of the cellulose microfibrils.
c) The variables introduced by the light source include intensity and wavelength/filtration. Little has been reported about the effect of specific wavelengths on aqueous bleaching of paper. 8 Hon has reported success at bleaching mixed pulp paper with a narrow band wavelength of 360-370 nm (below 360 run damage occurs) for 36 minutes with a neutral distilled water spray at 25-30ºC. He found this increased brightness from below 70 to 89 at 200 watts. With newsprint he recommends using 360 and 540 nm frequencies separately, since the 360-370 run range can cause chromophore formation. 9
1.2.2 Despite extensive research, the fact remains that much is still not understood about cellulose chemistry. Baugh noted that the basic information on the absorption spectrum of the compound which is photolyzed has not been obtained for cellulose [8]. Ergerton observed that "the complications produced by the presence of oxygen and water vapor are not fully understood" [21]. For example, moisture can both inhibit and accelerate degradation of paper, as noted by Phillips, who goes on to assert that "the mechanism of direct photolytic degradation of cellulose has not been clarified, and presumably will not be until the initial light absorption process is better understood" [53].
1.2.3 A wide selection of tests have been used to detect and measure changes in paper. Tests for mechanical properties include fold endurance 10 and tensile measurements of the strength of the paper network (standard span tensile test), fiber (zero span) or internal bonding (z-direction tensile test). Changes in molecular weight can be detected by viscosity for bulk measures of molecular weight, and gel permeation chromatography for molecular weight distribution. Changes in molecular structure can be determined by carboxyl content through titration or iodimetric measurements, or carbonyl content measured by hot-alkali-solubility, copper number, etc. Raman Spectroscopy and Nuclear Magnetic Resonance can also determine carbonyl and carboxyl content as well as ester and ether content. However, it is possible that some properties might be too subtle to be detected by mechanical, physical, or chemical tests. For instance, a decrease in degree of polymerization of cellulosic fibers from 4,400 to 800 (which represents about 4 molecular chain cleavages) does not "measurably change the physical properties of fibers, such as breaking strength and elongation-at-break," as noted by Phillips [53].
1.2.4 Interpretation of data is often complicated by multiple reactions. For example, color reversion, found by Savard in 1986, could be attributed to many things. Hon postulated 14 theories for brightness reversion of bleached pulps, including residual lignin, furfural, reductone, resin, poor washing, pH, metallic catalysts and resinates, diffusion, carbonyl groups, water impurities, microorganisms, low bleach residual and ultraviolet radiation [42]. Reversion may be thermally induced but photolytically increased, as Silvy observed in his experiment whereby cellulose, yellowed by heat for 3 months and then light bleached 70 hours, underwent greater yellowing after subsequent dark thermal ageing for 25 days [58]. Color reversion also occurs with chemical bleaching, as noted by Burgess [14]. Stiffening, found by van der Reyden in 1981, could also be caused by a variety of factors. Wosniak noted that stiffness can result from cross-linking, which "can occur following the oxidation of hydroxyl groups to aldehyde groups ...ether linkages could be formed between hydroxyl groups of adjacent chains by the elimination of water" [60]. Cross-linking of cellulose or its derivatives can be induced by light irradiation below 360 nm, although other wavelengths might cross link sizing materials. As noted by Ranby et al. short term irradiation produces a drop in water retention and moisture regain, either because cross-linking forms water resistant bonds, or because such irradiation forces amorphous or disordered regions, which are accessible to water, to become more ordered and crystalline, consequently water inaccessible [54].
| GROUP I UNTREATED | GROUP II Mg (HCO3)2 | GROUP III SUNBLEACHED & Mg (HCO3)2 | |||||||
| * Paper Type | Range | Mode | Average | Range | Mode | Average | Range | Mode | Average |
|---|---|---|---|---|---|---|---|---|---|
| I | 7-9 | 7 (60%) | 7.5 | 6-8 | 8 (50%) | 7.3 | 7-9 | 7 (40) % | 7.9 |
| II | 6-9 | 6 (50%) | 7.1 | 6-7 | 6/7 | 6.5 | 5-8 | 8 (40% ) | 7.0 |
| III | 9-10 | 9 (70%) | 9.3 | 9-10 | 9 (90%) | 9.1 | 9-10 | 10 (60%) | 9.6 |
* Paper Types:
I: primarily unbleached mixed bast fibers with some coniferous
chemical pulp.
II: rag paper of highly mascerated cotton linters and flax, with
some bast fibers.
III: bleached and unbleached grass and straw with some cotton and
chemical pulp.
Fig. 2. Sunlight vs. Artificial Light Sources
A Comparison of Relative Spectral Energy Distribution
Sunlight
[unreadable] Average Optimum Direct Global Radiation
Measured [45' 3. 3 20 84]?
Sunshine Carbon Arc
As used in Atlas Weather-Ometer. Cores D Filtered
Xenon Arc Lamp
As used in Atlas Weather-Ometer. 6500 Watt Xenon Lamp with Borosilicate: inner and Outer filters 340 nm control [35 watt [unreadable" m3 ?]
-FS-40 Fluorescent Sun Lamp
Is commonly used in the Atlas [UVCOM ?] [ureadable[
Accelerated weathering devices are used to determine the effects of sunlight on various Substrates.
This graph illustrates the spectral energy distribution as a function of the wavelength produced by a number of artificial light Sources The farther left the wavelength appear on the graph (i.e.. shorter wavelength). the higher the energy output generated. The graph compares these energy outputs to terrestrial sunlight The closer the energy distribution to sunlight the more reliable and accurate the results of the experiment. Accelerated weathering devices that emit larger amounts of shorter wavelengths cause samples to fail in shorter periods of time. and often correlate less well than those instruments which emit wave lengths closer to the distribution of terrestrial sunlight.
CIBA-GEIGY
1.2.5
Finally, criteria need to be established to determine whether measured changes are significant or not in terms of benefit or detriment to paper. If a certain degree of stiffening is considered significant, for instance, we must then determine whether or not such stiffening is beneficial to paper. For example, Baugh noted that the frequency corresponding to the energy needed to cause chain scission in cellulose is in the ultraviolet end of the spectrum at 340 nm or less (equivalent to a bond energy of c. 340 kJ Mol-1). During the initial chain scission, photodegraded cellulose has low molecular mass sugars like glucose and oligosaccharides [9]. Atalla, however, observed that lower molecular weight cellulose, in the presence of moisture, is more mobile and susceptible to ordering, leading to crystallization, decreasing elasticity, and brittleness. Moderate cross-linking could increase strength by extending the "domain spanned by covalently linked molecular entities to counteract the reduction resulting from chain scission" but "if carried too far it could result in embrittlement" [4]. In addition, Scallan noted cross links inhibit swelling as acid groups within fibers tend to increase pressure generated by counterions until "osmotic pressure within the cell wall is reduced by dilution and the pressure equals the structure's resistance to further expansion" [57]. Obviously the consequences of these changes depend on many other circumstances involving the function of the paper and its environment. Reactions like chain scission and cross-linking, which are both induced by light irradiation of dry paper, could counteract each other, making detection and interpretation difficult.
2. Purpose
Given the above, it is understandable that questions about aqueous light bleaching remain. At the Conservation Analytical Laboratory, we have conducted since September 1985 a series of studies to address several of the variables associated with the treatment. To understand the effect of various light sources on aqueous light bleaching, separate projects have investigated wavelength efficiency, using Oriel Long Pass filters exposed to sunlight and Tungsten light, and wavelength specificity, using Microcoatings Narrow Band Pass filters exposed to xenon arc light. To understand the mechanism of aqueous light bleaching, investigations have been conducted into the role of oxygen. Other areas of interest include changes in viscosity, the relationship of alkalis and swelling agents, and the interference of paper sizes or coatings. For the purposes of the present paper, three comparative studies will be described. The conclusions we draw from these initial experiments are by no means final or definitive. It is hoped that awareness of this material will stimulate further and more conclusive experimentation by other researchers in the field. The three most recent studies are as follows:
2.1 Studies
STUDY I
A experiment to separate the effects of aqueous light bleaching on paper from the effects achieved by dry exposure and dark immersion over long time periods, to provide controls for different reaction mechanisms resulting from some of the variables.
Fig. 3.
Fig. 4.
STUDY II
A comparison of aqueous light bleaching with light bleaching in a solvent system using ethanol, since the use of solvents could expand options for non-aqueous bleaching of water sensitive objects.
STUDY III
A comparison of aqueous light bleaching to chemical bleaching systems using sodium borohydride and hydrogen peroxide, since ultimately we must determine the effectiveness and hazards of light bleaching in relationship to chemical bleaching.
2.2 Experimental
For each of these studies, the following conditions remained constant:
Light source: An Atlas Ci35W Controlled Irradiance Xenon Arc Exposure System (Weather-Ometer), 11 with a filtered spectral output simulating average optimum Miami daylight from 9:00 am to 3:00 pm (Fig. 2). 12 Filtration resulted from both the borosilicate filters on the lamp (Fig. 3) and the polystyrene bottles (Fig. 4), which combined to eliminate most ultraviolet radiation below 360 nm.
Immersion containers: 600 mL polystyrene culture flasks which attached to the specimen rack in the Weather-Ometer with bottle mounts.13 13
Sample paper (Fig. 5):
1. an evenly discolored, naturally aged mixed pulp paper, identified
as Strathmore Quality and analyzed by the Institute of Paper
Chemistry as primarily rosin sized, chemically treated softwood with
some hardwood and cotton.
2. Whatman filter paper #6, having characteristics similar to the
Strathmore paper in fiber make-up, but without any size.
3. Whatman filter paper #1, made of cotton seed hair, also unsized.
14
All samples except untreated controls were prewashed in dilute calcium hydroxide, pH 9, for 4 hours to remove alkaline soluble degradation and discoloration products. 15
Colorimetry was undertaken with both a Minolta Chroma Meter 100 16 and a HunterLab Ultrascan Spectrocolorimeterl7 using the CIE L*a*b* color notation system, where L* represent value (ranging from 100 as white and 0 as black), a* and b* represent hue and chroma, with a* representing the degree of redness (if positive ) or greenness (if negative) and b* representing yellowness (if positive) or blueness (if negative).
RH measurements were made with a Corning Model 12 Research pH meter using a Beckman D90 flat head electrode for the paper samples, and using a solution electrode for the immersion solutions.
Tensile measurements were made using an apparatus designed by Marion Mecklenburg. 18
3. Study I. the Effects of Exposure and Immersion Times on Tensile strength of paper.
3.1 Procedure
The purpose of the procedure was to correlate exposure time to change in color and mechanical strength, and to monitor changes in pH and temperature over time, to determine whether these factors could be correlated in any way to change in color and mechanical strength of immersed samples. For each of the three paper types listed in Fig. 5, the following preparation and procedure occurred. Forty 4x4½" samples were cut, with 8 set aside as untreated controls. The remainder were pretreated by washing in a dilute solution of calcium hydroxide (pH 9) to remove any alkaline soluble discoloration products. All forty samples were then placed in polystyrene bottles to be inserted into an Atlas Weather-Ometer C135 under 5 different conditions (Fig. 6): untreated and dry, protected from the light by an aluminum foil wrapping about the bottle; prewashed and dry, protected from the light with foil; prewashed and immersed in a dilute calcium hydroxide solution (pH 10), protected from the light with foil; prewashed and immersed in the same solution but exposed to xenon light; and prewashed and dry but exposed to the light. Time in the Weather-Ometer ran 2, 4, 6, 8, 24, 48, 72, and 96 hours. 19
There are several problems inherent in using the Weather-Ometer for light bleaching of paper in aqueous solutions. Solutions must be in containers, and unless these containers are made of quartz, they will alter the light spectrum. Also, temperature and pH within the bottles are almost impossible to control once the bottles are placed in the Weather-Ometer, especially over a long period of time.
The ambient temperature within the Weather-Ometer ranged between 24.5 and 35.2ºC from the beginning to the end (after 96 hours) for each of the three papers. The ambient temperature of the dry samples within the bottles, as measured with a thermocouple as the bottles were removed from the Weather-Ometer, reached 31ºC. It should be assumed that the highest temperature recorded would reflect the subsequent temperatures. The temperature of the immersed samples kept in the dark went from about 31.5ºC to 33ºC after 4 .hours, gradually leveling off to about 34ºC after 72 hours in both Whatman samples (Fig. 7). In the Strathmore sample the increase was slightly greater (from 31 to 35.5ºC) and more consistent, increasing an average of about 0.05ºC with each time increment (Fig. 8). (Deviations resulted from the immediate cooling mechanism within the Weather-Ometer which is activated as soon as the door is opened, causing a dramatic drop in temperature within the few minutes required to remove samples) The temperature of the immersed samples exposed to light ht ran about 7ºC higher for the Whatman samples (ranging from 39 to 41 C), and about 5ºC higher for the naturally aged Strathmore (from 36.5 to 39ºC). In summary, depending on the condition, the ambient or solution temperature within the bottles ranged from 31 to 41ºC. 20
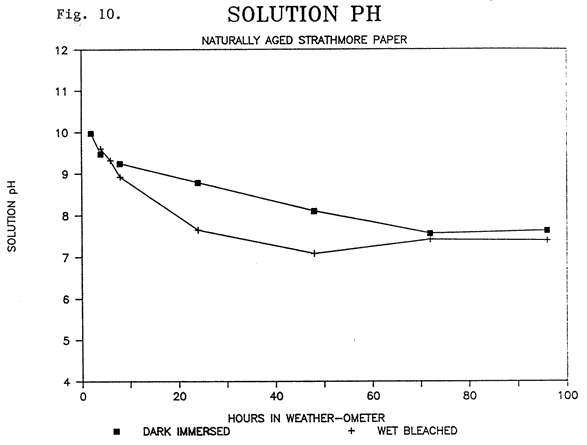
The immersed samples were in a solution of calcium hydroxide, and the pH of the solution containing the undegraded Whatman papers, whether in the dark or not, remained steady at about 10 until after 24-48 hours, at which time the pH dropped, by 96 hours, to 8 for the light exposed immersion, and 9 for the dark immersion (Fig. 9). Again, the naturally aged Strathmore paper had a consistant decline in pH from 10 to about 7.4 after 48 hours regardless of whether the samples were in the light or dark (Fig. 10). Although there was a sharper decline in the light bleached, immersed sample, especially after 8 hours, the drop in pH can not be strictly related to the influence of light since a similar drop occurred with the samples immersed in the dark. That the change in pH has more to do with degradation products and initial acidity is further supported by the fact that, as noted, the new Whatman papers did not experience a drop in pH until they were immersed for 48 hours, and again the drop was consistent for samples immersed in both the light and dark.
Fig. 7. AMBIENT TEMPERATURE IN BOTTLE
Fig. 8. AMBIENT TEMPERATURE IN BOTTLE
Fig. 9. SOLUTION PH
Fig. 10. SOLUTION PH
The actual pH of the paper samples themselves showed slight changes: the naturally aged, chemical pulp, Strathmore papers decreased slightly in pH on the whole, dropping from about 7 for the prewashed samples only about one unit, except for the dry bleached sample which dropped about 2 units. The untreated Strathmore remained at about 5. It is interesting to note that dry exposure to 96 hours of light cause the prewashed Strathmore sample to return to its original, untreated, pH level of 5. The pH of the chemical pulp Strathmore and Whatman 6 papers decreased slightly from about pH 7 for the prewashed samples to 5 for the dry bleached samples, while the Whatman #1 (cotton) samples averaged 7 regardless of the findings. The chemical pulp Whatman #6 dry bleached samples' pH dropped 1½ units from 7 to 5.5 while the wet bleached samples' pH increased 1½ units from 6.1 to 7.5. The pH of the remaining samples averaged about 6. Regardless of condition, the pH of the Whatman #1 samples averaged 7 throughout.
3.2 Findings: Colorimetry and Tensile Measurements
3.2.1
Colorimetric readings were taken with a Hunter Labs Ultrascan Spectrocolorimeter in CIE L*a*b*. The L* measurements, which reflect the value or lightness (+) or darkness (-) of the sample indicate that for both the Whatman #6 and naturally aged Strathmore samples, the greatest lightening takes place within the first two hours, and increases most, particularly in the naturally aged Strathmore sample, up to 8 hours, leveling off after 24 hours (Figs. 11 and 12). A similar increase is not seen in the immersed samples kept in the dark, indicating that light is the dominant force in lightening. Surprisingly, only a slight darkening occurs in the prewashed, dry exposed naturally aged samples, and then only after 8 hours of exposure.
The a* measurements, which are an indication of shifts to red (+) or green (-) may reflect upon the lack or presence of degradation products (Figs. 13 and 14). The new Whatman samples appear to have a slight increase in red in both the wet and dry bleached samples especially, which might indicate the formation of photo degradation products. The slight increase in the immersed sample might indicate possible thermal degradation products resulting after 48 hours. In the naturally aged Strathmore samples, the opposite seems to be happening, with a loss of red over time in all samples, especially the wet bleached and dry bleached ones. This possibly indicates that if degradation products already exist, their reduction with light exposure or immersion exceeds the formation of new products.
Fig. 11. L*a*b* COLORIMETRY AFTER WEATHER--OMETER
Fig. 12. L*a*b* COLORIMETRY AFTER WEATHER--OMETER
Fig. 13. L*a*b* COLORIMETRY AFTER WEATHER-OMETER
Fig. 14. L*a*b* COLORIMETRY AFTER WEATHER-OMETER
Fig. 15. L*a*b* COLORIMETRY AFTER WEATHER-OMETER
Fig. 16. L*a*b* COLORIMETRY AFTER WEATHER--OMETER
The b* measurements, which indicate shifts to yellow (+) or blue (-) or loss of yellow, again show the greatest loss of yellow occurring in the first two hours and continuing, with greater magnitude in the naturally aged sample, over the 96 hours (Figs. 15 and 16). While immersion does cause loss of yellow, the degree appears again to be a direct result of the light, as the dark immersed samples lose yellow at lesser rate. This is probably not the effect of temperature, as the ratio of temperature increase to lightening is considerably different between the dark and light immersed samples. (That is, the L* and b* of the Strathmore dark immersed samples changes by 2 units while the temperature changes by 4.5ºC, while the light immersed samples change by 2-3 units, with a temperature change of only 2ºC. Likewise, it probably is not a pH related phenomenon since the pH goes down, and again, with the Whatman 6 papers, only after some 24-48 hours, while the Whatman 6 L* and b* change consistently in the wet bleached samples). 21
To summarize, in this study, two hours of exposure to the xenon arc lamp (2.4 kJ) caused the greatest color change in the CaOH immersed samples, regardless of the paper composition. 2222Exposures exceeding 24 hours under these conditions serve little purpose colorimetrically. Prolonged immersion in calcium hydroxide solution alone continues to lighten the paper but at no point does it reach the level obtained in the combination with light. However, although there is a great difference colorimetrically between samples aqueously bleached between 2 and 96 hours, little difference in tensile properties was detected among samples immersed from 2-96 hours regardless of whether that immersion was in the dark or exposed to xenon radiation, as indicated by the findings below. 23
3.2.2 Tensile Measurements for the Whatman 6 paper, based on the mean strains, indicate that calcium hydroxide washing increases the strain to failure over an untreated sample, but no effects can be distinguished as a result of exposure time, light exposure, or whether the sample was dry or immersed (Figs. 17a and-b). Based on the mean stresses (Figs. 18a and b), the findings indicate that 1) washing in calcium hydroxide reduces strength; 2) further immersion reduces strength additionally, as in the case of the samples immersed in the light and dark (distinction between these two groups can not be made, so that the loss of strength may be as much from immersion or temperature as from exposure to light); 3) dry light exposure reduces strength as compared to dry exposure in the dark; 4) no distinction can be made with respect to length of exposure.
For the naturally aged Strathmore papers, the mean strains (Figs. 19a and b), indicate the same trend as the Whatman #6 papers. Based on the mean strain alone, washing in calcium hydroxide increases toughness and flexibility. Strengths do not vary significantly under any circumstances (Figs. 20a and b).
To summarize, the results of this study demonstrate little effect on tensile properties by light as compared to prewashing alone. This may suggest that the amount of rebonding that occurs upon washing far exceeds the number of bonds broken upon subsequent light exposure. 2424The fact that subsequent exposure did not significantly alter the tensile properties after the initial immersion corresponds to findings by Annis and Reagen who found no physical or chemical change in breaking load and elongation of (cotton) fabric regardless of the exposure times between 4 and 32 hours [1].
Fig. 17a.
Fig. 17b.
Fig. 18a.
Fig. 18b.
Fig. 19a.
Fig. 19b.
Fig. 20a.
Fig. 20b.
4. Study II. Comparison of Light Bleaching Aqueously and in Ethanol.
4.1 Procedure
Samples of naturally aged Strathmore papers, prewashed as noted above, were immersed in a dilute calcium hydroxide solution (pH 10) and a 9:1 ethanol/deionized water solution and placed in the polystyrene bottles in the Weather-Ometer for 24 hours (totaling 30.2 kilojoules/square meter of exposure).
4.2 Findings: Colorimetry and Tensile Measurements
4.2.1
Fig. 21. L*a*b*: WATER VS ETHANOL WITH LIGHT
Fig. 22. L*a*b*: WATER VS ETHANOL WITH LIGHT
Fig. 23. L*a*b*: WATER VS ETHANOL WITH LIGHT
Compared to untreated and washed only samples, both water and ethanol achieved a lighter value (L*) (Fig. 21), and chromatically shifted toward negative readings, in the direction of green (loosing red) in the a* (Fig. 22) and blue (loosing yellow) in the b* (Fig. 23). This suggests that water soluble items could be effectively light bleached in water/ethanol solutions.
4.2.2
Fig. 24. AQUEOUS LIGHT BLEACHED 24 HRS
Fig. 25. ETHANOL LIGHT BLEACHED 24 HRS
Preliminary tensile tests indicate that 90% ethanol might result in a slightly lower strain to failure in the paper, i.e. a loss of toughness and elasticity (Figs. 24 and 25).
5. Study III. Comparison of Light Bleaching to Chemical Bleaching.
5.1 Procedure
Samples of naturally aged Strathmore paper, prewashed as noted above, were immersed for two hours each in the chemical bleaches sodium borohydride (0.3%) and hydrogen peroxide (3%), and in an alkalinized aqueous solution exposed to light.
5.2 Findings: Colorimetry and Tensile Measurements
5.2.1
Fig. 26. L*a*b*: LIGHT VS CHEMICAL BLEACHING
Fig. 27. L*a*b*: LIGHT VS CHEMICAL BLEACHING
Fig. 28. L*a*b*: LIGHT VS CHEMICAL BLEACHING
Two hour immersions, in the dark, in sodium borohydride (0.3%) and hydrogen peroxide (3%), were comparable to a two hour aqueous light bleached sample at 2.4 kJ/m . While all readings were close, hydrogen peroxide cause the greatest lightening in the L* value (Fig. 26), with light and sodium borohydride close: the same occurs in the a* (Fig. 27), but in the b* (Fig. 28) sodium borohydride had the greatest loss of yellow. Light seemed to be the least effective colorimetrically in this two hour study. 25
5.2.2
Fig. 29 AQUEOUS LIGHT BLEACHED 2 HRS
Fig. 30. SODIUM BOROHYDRIDE 2 HRS
Fig. 31. HYDROGEN PEROXIDE 2 HRS
Preliminary tensile measurements indicate, however, comparable results between samples treated by light (Fig. 29) and sodium borohydride (Fig. 30). Those treated with hydrogen peroxide had reduced strains to failure (Fig. 31).
6. Conclusion
6.1 Colorimetry of above 3 studies:
Two hours of exposure to the xenon arc lamp (2.4 kJ) caused the greatest color change in the CaOH immersed samples, regardless of the paper composition. Lightening continued far more slowly after two hours, and tapered off at 24 hours. Consequently, the most effective bleaching occurs within the first two hours, and bleaching longer than 24 hours under these conditions serves little purpose colorimetrically. The same holds true for prolonged immersion in a dilute calcium hydroxide solution in the dark (similar to washing without changing the bath water), but the degree of lightening is less then half, and at no point achieves the visual effectiveness of aqueous light bleaching. (i.e. in our samples, 48-96 hours of immersion in a dilute calcium hydroxide solution still does not produce the degree of lightening of 2 hours of aqueous light bleaching).
The degree of bleaching achieved in aqueous light bleaching with a xenon arc lamp is fairly comparable to chemical bleaching with sodium borohydride (0.3%) and hydrogen peroxide (3%) for the same 2 hour time period. The same is true with 9:1 ethanol/water and a dilute calcium hydroxide solution for 24 hours.
6.2 Tensile measurements of the above 3 studies:
The greatest change in tensile strength occurs not over an extended time period (i.e. no significant difference between 2-96 hours regardless of whether the sample was exposed to light or immersed in solution) but rather between untreated and washed samples. Once our samples were washed, it no longer matter a great deal whether they were then immersed up to 96 hours or exposed to light either immersed or dry.
Two hours of aqueous xenon light bleaching showed no significant difference from 2 hours of sodium borohydride (0.3%) in terms of stress/strain curves, and did show a greater degree of strength than hydrogen peroxide (3%) bleaching over the same time period. Light bleaching in ethanol/water 9:1 showed slightly less strength than a dilute calcium hydroxide solution alone.
7. Acknowledgments
Thanks go to the many people who consulted on, and helped with, many aspects of the present project, including the CAL Science Staff, CAL information officers Marge Cleveland and Sherry Medley, and MSC librarian Karen Preslock.
8 Endnotes
1. Keyes, K.M. "Alternatives to Conventional Methods of Reducing Discoloration in Works of Art on Paper," Preprints. Cambridge 1980 International Conference on the Conservation of Library and Archive Materials and the Graphic Arts, Institute of Paper Conservation, UK, p.170.
2. van der Reyden, D. "Wax Pick Testing: A Preliminary Study," Art Conservation Training Programs Conference Papers, New York University, March 26-27, 1981. This study, to measure the surface strength of light bleached papers, was undertaken under the supervision of Marjorie Cohn.
3. Ibid. "Sun Bleaching Project," Unpublished Manuscript, Spring, 1981.
4. Branchick et al. compared 18th C. rag and 20th C. alpha cellulose papers exposed in deionized water and magnesium bicarbonate solutions to sunlight (6400 footcandles, 80ºF (26.4ºC), 4 hrs), fluorescent light (8 GE 100 watt Power Groove Cool White, 96ºF (33.3ºC) for a total of 16 hrs), and black light (40 watts, 105ºF (40.5ºC} 16 hrs). A Bausch & Lomb Spectronic 600 Spectrophotometer with integration sphere reflectance accessory recorded reflectance at 436, 546, & 700 nm MIT Fold endurance by the Institute of Paper Chemistry showed loss in strength in the rag paper.
5. Savard exposed 2 modern papers (one with 50$ cotton content, the other with higher wood pulp content), thermally aged at 80ºC and 50% RH for 31 days, to 14 GE Cool White fluorescent tubes with UV filtering sleeves for 1,2,5,10, & 24 hrs dry; in magnesium bicarbonate and citrate solutions; calcium sulfate; and ammonium hydroxide solutions, at 34ºC. Reflectance (Macbeth 1500 Colorimeter, using Delta E between the sample and standard) showed color reversion after re-aging, predominately in dry and magnesium bicarbonate exposures.
6. Robert Feller reported on Oct. 6, 1988 at the Canadian Conservation Institute Symposium '88 in a presentation entitled "Bleaching by Light: Studies on the Bleaching of Thermally Discolored Sugars and Other 'Model' Compounds" his findings in reference not only to xylose and arabinose, but also ribonose, dextriol, and mannitol, among other things. His paper is to be published in the post prints of the symposium.
7. Feller, at the same conference cited above, Oct. 6, 1988, in a presentation entitled "Bleaching by Light: Effect of pH on the Bleaching or Darkening of Paper Both in the Dry and Immersed Condition Under Visible and Under Near Ultraviolet Radiation," noted that even slightly alkaline solutions increased the speed of light bleaching.
8. Wavelength studies on dry paper include Feller, R. et al., "The Darkening and Bleaching of Paper by Various Wavelengths in the Visible and Ultraviolet," AIC Book and Paper Postprints, Milwaukee, May 1982, p. 65ff. For dyed, dry paper substrates, see McLaren, K. "The Spectral Regions of Daylight which cause Fading," Journal of the Society of Dyers and Colourists, 1956, 72, pp. 86-99. Other studies are concerned with cellulosic fabrics, as in Flynn, J. et al., "Cellulose behavior with Filtered Sunlight," Textile Research Journal, 18, 1948, pp. 350-357.
9. Private communication, Spring, 1987.
10. For a discussion on the problems relevant to testing, especially in respect to fold endurance, see Antoinette Dwan's article, "Paper Complexity and the Interpretation of Conservation Research," JAIC, Vol. 26, No. 1, Spring 87, pp. 1-18.
10. The Atlas Weather-Ometer has a 3500 watt water cooled long arc xenon lamp, with the automatic digital setpoint in W/m2 at 340 nm, measuring the radiant exposure time in kilojoules/m2 hours, programmed to continuous light. Black panel temperatures, set at 0, did not remain constant, but rather increased from a minimum of 29.8 to a max of 53.3ºC over 96 hours; wet bulb ranged from a min. of 16.8 to 23.5 and dry bulb ranged from 24.5 to 35.2ºC. The total irradiance in kilojoules for 2 hr. exposures was 2.4; for 24 hours 30.2; and for 96 hours 122.0-2 (/m2).
12. Xenon light sources have been used by references 9, 23, 25, 26, and 31, although not necessarily the same kind.
13. The polystyrene falcon tissue culture flasks, with phenolic screw caps, are from Fisher Scientific, catalog # 08-772-1a. Quartz bottles would be best for their compositional and spectral purity, but were prohibitively expensive for the initial experiments.
14. For the photodegradation products of Whatman filter paper #1, see Phillips [53], who exposed Whatman 1 to unfiltered light and got low molecular weight sugars: D-glucose and related oligosaccharides. The main volatile products were CO, CO2, and H2, and acetaldehyde, propionaldehyde, methyl formate, acetone, methanol, ethanol, and ethane, all detected by gas chromatographic retention times.
15 Phillips [53] notes that alkali pretreatment of cellulose influences rates of gas evolution.
16 This is a reflected subject-color colorimeter with a pulsed xenon light source; 6 silicon photocells (for double-beam feedback system)´ filtered to detect primary stimulus values for red, green, and blue light; a d/0 illuminating system, and a 0.08 mm measuring area. It was set for CIE Illuminant D65 (6504K) and calibrated on a Minolta standard-white reflector plate.
17. Instrument Std. D80, standardization date February 1988, calibrated to white tile standard #7520W.
18. For information concerning this apparatus, see Marion F. Mecklenburg, "The Role of Water on the Strength of Polymers and Adhesives," Doctoral Dissertation, University of Maryland, 1984.
19. 70 hours is often used in Xenotests. According to Ranby [54], 100 hours in a Type XI Atlas Weather-Ometer approximates one year outdoor exposure. According to Friele [31] 2000-2500 hours in xenon equal one years coastal exposure.
20. Branchick et al. [12] registered temperatures of 40.5ºC for solutions holding samples and exposed to ultraviolet light; 33.3ºC for those exposed to the GE Power Pack fluorescent lamps, and 26.4ºC for the sun-exposed sample solutions.
21. Hon [42] notes that dry paper yellows on exposure to light because of fats, resins, waxes, glues, gelatin, or rosin discoloring.
22. A kilojoule is a measurement of energy equal to 107 erg or app. 0.239 calories, 0.000948 BTU or 0.000278 watt-hour (1 watt second). Afoot candle equals 15.83 erg/cm2sec at 555 nm. A lux (illuminance) equals 1.471 erg/cm2sec at 555 rum. Cellulosic materials have activation energies from 100-125 kJ/mol (protein 125-170).
23. In respect to stress/strain curves of tensile measurements, stress is an indicator of strength in terms of the force required to pull a sample apart, and strain is an indicator of toughness in terms of elasticity and flexibility of a sample. Below is an example of a stress/strain curve for Whatman #6 samples:
(I) represents the untreated controls and (II) represents samples prewashed in dilute calcium hydroxide solution (pH 9) for four hours. The difference in stress (A) indicates that the CaOH washed samples in group II registered a decrease in stress to failure which suggests lower tensile strength in terms of the force required to pull the samples apart. The difference in strain (B) indicates that the CaOH washed samples registered an increase in strain to failure, suggesting a greater toughness in terms of elasticity and flexibility
24. The dramatic change detected upon first immersion of the paper may be because, as Baum notes, during alkaline pulping, the paper stock is in its most swollen state and consequently upon drying the strongest hydrogen bonding occurs. As paper degrades, bonds break. Subsequent water immersion and swelling, while breaking some additional bonds and rendering paper weaker when wet, upon redrying, form new hydrogen bonds exceeding the number which had survived in the original degraded paper [10].
25. Annis et al. compared hydrogen peroxide and sodium carbonate for .5 to 3 hours to dry sunlight bleaching for 4, 16 and 32 hours. The greatest effect occurred after 3 hours bleaching in hydrogen peroxide or 32 hours bleaching in sunlight for 19th C. cotton fabric using AATCC Test Method 110-1975, Reflectance, Blue, and Whiteness of Bleached Fabrics. They found no significant physical or chemical changes in terms of breaking load and elongation. They noted the greatest sun bleaching in the first 4 hours, with less change between 16 and 32 hours. They felt 16 hours of dry sun bleaching was comparable in appearance to 3 hours bleaching in hydrogen peroxide (6%).
9. Selected Bibliography
(The following have been selected from a bibliography of 190 references which have been collected and partially excerpted and is available upon request. It is intended to provide an introduction to the various topics related to the studies outlined in this paper, subdivided, for instance, as follows: photodegradation [8-9, 11, 18, 20, 23, 25-31, 38-42, 50-55, 57-58, 60]; filtration/wavelengths [5, 7, 9, 18-19, 24, 26, 30, 34, 38, 47, 51, 53, 56]; chain scission and cross-linking [9, 18, 27, 29, 57, 60]; water [1-2, 4, 8, 9-10, 16-I8, 21, 39, 42, 47, 49, 54-56, 60]; alkali [2, 10, 27, 32, 42, 43, 53, 56, 60]; solvents [10, 16, 17]; swelling [9, 10, 32, 39, 43, 57]; rosin [42, 45-46, 55]; aging [3, 24, 27, 33, 35, 42, 56, 58, 60]; temperature [17, 19, 21, 33-35, 40, 54, 58, 60]; oxygen [2, 3, 9, 17, 18, 21, 35, 42, 46-47, 54, 60]; aqueous light bleaching [5, 6, 12, 22, 44, 49, 56, 59]; chemical bleaching [15, 36, 37]; hydrogen peroxide [1, 2, 17, 60]; tensile testing [4, 7, 23, 35]; viscosity and degree of polymerization [7, 13, 32, 35, 42, 47, 49, 53, 60]; hot-alkali solubility [27, 48]; color reversion [14, 50, 56]; reflectance [35, 50, 56, 58].)
1. Annis, A.K. and B.M. Reagan, "Evaluation of Selected Bleaching Treatments Suitable for Historic White Cottons," Studies in Conservation, 24, 4, November, 1979, pp. 171-178.
2. Ardon, M., Oxygen. Elementary Forms and Hydrogen Peroxide, W.A. Benjamin, New York, 1965.
3. Arney, J.S., "Accelerated Aging of Paper, the Relative Importance of Atmospheric Oxidation," TAPPI, 62, 7, July 1979.
4. Atalla, R.H., "Crystallinity of Cellulosic Fibers," Preservation of Paper and Textiles, II, ACS, 1981, pp. 169-176.
5. Baker, C., "The Double-Sided Light Bleaching Bank," AIC Book and Paper Group Annual, 4, 1986, pp. 88-91.
6. -----,"Practical Methods for Sun and Artificial Light Bleaching Paper," AIC Book and Paper Group Postprints, Milwaukee, Wisconsin, May 1982, p. 14.
7. Barr, G. and I. Hadfield, "The Nature of the Action of Sunlight on Cotton," Journal of the Textile Institute, 18, No: T, 1927, pp. 490-3.
8. Baugh, P.J. and G.O. Phillips, "Photochemical Degradation," Cellulose and Cellulose Derivatives, eds. Norbert M. Bikales and Leon Segal, Part V, Wiley-Interscience, NY, 1970, pp. 1047-1078.
9. Baugh, P.J. "Photodegradation and Photooxidation of Cellulose," Developments in Polymer Photochemistry-2, ed. Norman S. Allen, Applied Science Publishers Ltd., London, 1971, pp. 165-214.
10. Baum, G.A. "Moisture and Bonding" Unpublished Manuscript, Institute of Paper Chemistry.
11. Bos, A. "The Ultraviolet Spectra of Cellulose and Some Model Compounds," Journal of Applied Polymer Science, 16, 1972, pp. 2567-2576.
12. Branchick, T., K. Keyes and C.F. Tahk, "A Study of the Bleaching of Naturally Aged Paper by Natural and Artificial Light," AIC Preprints, 1982, Milwaukee, p. 29 ff.
13. Burgess, H.D., "Chemical Analysis of Paper, Session II," AIC Book and Paper Specialty Group Papers, Baltimore, 1983.
14. -----, "The Colour Reversion of Paper After Bleaching," Preprints. Cambridge 1980 International Conference on the Conservation of Library and Archive Materials and the Graphic Arts, Institute of Paper Conservation, UK, 1980, pp. 171-183.
15. -----, and J.F Hanlan, "The Degradation of Cellulose in Conservation Bleaching Treatments," J. IIC-Canadian Group, 4, 2, 1979, pp. 15-22.
16. Graver, J.K. and D.L. Taylor, "Ultrasonic Impedometric Studies in the Cellulose Pulp/Water System," Consolidation of the Paper Web, I, ed. Francis Bolam, Technical Section of the British Paper and Board Makers' Association (Inc.), London, 1966, pp. 445-472.
17. Daniels, V., "A Photographic Method for Detecting the Oxidation of Materials," 7th Triennial Meeting Preprints, ICOM Committee for Conservation, Copenhagen, Sept. 10-14 1984.
18. Daruwalla, E.N., A.P. D'Silva and A.C. Mehta, "Photochemistry of Cotton and Chemically Modified Cotton, Part I: Behaviour During Exposure to Carbon-Arc and Solar Radiations," Textile Research Journal, 1967, 37, 3, pp. 147-172.
19. Davis, A. and D. Sims, Weathering of Polymers, Applied Science Publishers, London, 1983.
20. Delides, C.G. et al., "Degradation of Cotton by Ionizing Radiations," Textile Research Institute, 1981, pp. 311-317.
21. Egerton, G.S., "Radiation Chemistry: Photochemistry of Cellulose in the Far Ultraviolet," Nature, June 9, 1962, 194, pp. 968-969.
22. Eldridge, B.P., "A Sun Bleaching Project," AIC Book and Paper Postprints, Milwaukee, May 1982, pp. 1-4.
23. Epps, Helen H., "Analysis of Fabrics Exposed to Light," Book of Papers, AATCC, October 5-7, 1983, New Orleans,pp. 271-279.
24. Erhardt, D., D. yon Endt, and W. Hopwood, "The Comparison of Accelerated Aging Conditions through the Analysis of Extracts of Artificially Aged Paper," AIC Preprints, Vancouver, 1987. pp. 43-55.
25. Feller, R.L., R.M. Johnston-Feller, and C. Bailie, "Determination of the Specific Rate Constant for the Loss of a Yellow Intermediate During the Fading of Alizarin Lake," JAIC, 25, 1986, pp. 65-72.
26. Feller, R.L, S.B. Lee and J. Bogaard, "The Darkening and Bleaching of Paper by Various Wavelengths in the Visible and Ultraviolet," AIC Book and Paper Postprints, Milwaukee, May 1982, pp. 65.
27. -----, "Relation of Cellulose Chain Scission to Hot-Alkali Soluble Content during Thermal and Photochemical Degradation in Paper," Journal of Imaging Science, 29, 2, March/April 1985, pp. 61-64.
28. -----, "The Kinetics of Cellulose Deterioration," Historic Textile and Paper Materials, ACS, 1986, pp. 331-347.
29. Flynn, J.H., "An Equation for Calculating the Number of Chain Scissions in the Photochemical Degradation of Solid Polymers," Journal of Polymer Science, 1958, 27, pp. 83-86.
30. -----, J.E. Sands, and K.S. Campbell, "Cellulose Behavior with Filtered Sunlight," Textile Research Journal, 1948, 18, pp. 350-357.
31. Friele, L.F.C., "A Comparative Study of Natural and Xenotest Exposure Conditions for Measuring Fading and Degradation," Journal Society Dyers and Colourists, 79, 1963, pp. 623-31.
32. Golova, O.P. and N.I. Nosova, N.I., "Degradation of Cellulose by Alkaline Oxidation," Russian Chemical Reviews, 42, 4, 1973, pp. 327-338.
33. Gray, G.G., "Determination and Significance of Activation Energy in Permanence Tests," Preservation of Paper and Textiles of Historic and Artistic Value, ed. John C. Williams, ACS, Washington, D.C. 1977, pp. 286-313.
34. Harrison, L.S., Report on the Deterioration Effect of Modern Light Sources, NY. Metropolitan Museum of Art, c. 1953.
35. Hernadi, S., "Thermal Ageing in Oxygen of Paper Made from Cellulose at Different Degrees of Beating," Sven. Papperstidn., 1976, 79, 13, pp. 418-423.
36. Hey, M, "Paper Bleaching: Its Simple Chemistry and Working Procedures," The Paper Conservator, 2, 1977, p. llff.
37. Holst, G., "The Chemistry of Bleaching and Oxidizing Agents," Chemical Reviews, 54, 1954, pp. 168-94
38. Hon, D.N.-S., "Formation of Free Radicals in Photoirradiated Cellulose. I. Effect of Wavelength," Journal of Polymer Science, Polymer Chemistry Edition, 13, 1975, pp. 1347-61.
39. .----, "Formation of Free Radicals in Photoirradiated Cellulose. II. Effect of Moisture," Journal of Polymer Science, Polymer Chemistry Edition, 13, 1975, pp. 955-9.
40. -----, "Formation of Free Radicals in Photoirradiated Cellulose. V. Effect of Temperature," Journal of Polymer Science, Polymer Chemistry Edition, 13, 1975, pp. 2363-2374.
41. -----, "Formation of Free Radicals in Photoirradiated Cellulose. VIII. Mechanisms," Journal of Polymer Science, Polymer Chemistry Edition, 14, 1975, pp. 2497-2512.
42. -----, "Yellowing of Modern Papers," Preservation of Paper and Textiles of Historic and Artistic Value II, Advances in Chemistry Series 193, ACS, Washington, D.C. 1981, pp. 119-142.
43. Katz, S., N. Liebergott and A.M. Scallan, "A Mechanism for the Alkali Strengthening of Mechanical Pulps," TAPPI, 64, 7, July 1981.
44. Keyes, K.M., "Alternatives to Conventional Methods of Reducing Discoloration in Works of Art on Paper," Preprints. Cambridge 1980 International Conference on the Conservation of Library and Archive Materials and the Graphic Arts, Institute of Paper Conservation, UK 1980.
45. Kimberley, A.E., and J.F.G. Hicks, "Light Sensitivity of Rosin Paper Sizing Materials," U.S. National Bureau of Standards Journal of Research, 1931, 6, pp. 819-827.
46. Launer, H.F. and W.K. Wilson, "Photochemical Stability of Papers," U.S. National Bureau of Standards Journal of Research, 1943, 30, pp. 55-74.
47. -----, "The Photochemistry of Cellulose. Effects of Water Vapour and Oxygen in the Far and Near Ultraviolet Regions," Journal of the American Chemical Society, 1949, 71, pp. 958-962.
48. Lee, S.B., J. Bogaard, and R.L. Feller, "Concerning the Exposure of Paper to Light: Discoloration of Handsheets of Known Initial Lignin and Hot-alkali-soluble Content," ICOM Committee for Conservation, 6th Triennial Meeting, Ottawa, 1981, pp. 81/14/4-1 to 7.
49. Lepage, M. and J. Perron, "Investigations of Some Aspects of the Light Bleaching of Paper," Student Papers Presented at the Art Conservation Training Programs Conference, May 2 and 3, The University of Delaware and the Winterthur Museum, 1985, pp. 54-68.
50. MacClaren, R.H., et al., "Brightness Reversion of Cellulose Exposed to Ultraviolet Light," TAPPI, 1962, 45, pp. 789-793.
51. McLaren, K., "The Spectral Regions of Daylight Which Cause Fading," Journal of the Society of Dyers and Colourists, 1956, 72, pp.86-99.
52. Padfield, T., "The Deterioration of Cellulose: The Effects of Exposure to Light, Ultraviolet and High Energy Radiation," Problems of Conservation, London, 1969, pp. 119-164.
53. Phillips, G.O., "Photochemistry and Radiation Chemistry of Cellulose," Cellulose Chemistry and its Applications, eds. T.P. Nevell and S. Haig Zeronian, John Wiley and Sons, NY, 1985, pp. 290-311.
54. Ranby, B, and J.F. Rabek, Photodegradation. Photo-oxidation. and Photostabilization of Polymers, John Wiley and Sons, NY, 1975.
55. Richter, G.A., "Relative Permanence of Papers Exposed to Sunlight," Industrial and Engineer Chemist, 1935, 27, pp. 177-185, 432-439.
56. Savard, G., "An Investigation of Whitening and Colour Reversion Effects on Light bleached, Artificially Aged Paper," Conservation Training Programs Student Papers, Conservation Center, Institute of Fine Arts, New York University, and Conservation Programs, School of Library Service, Columbia University, New York, April 30-May 2, 1986, pp. 57-83.
57. Scallan, A.M., "The Effect of Acidic Groups on the Swelling of Pulps: A Review," TAPPI, 66, 11, pp. 72-5.
58. Silvy, J. and J.F. Le Nest, "A Photochromic Effect Shown During the Ageing of a Cellulose Material by Heat and Light," Transactions of the Symposium, Cambridge, September, 1973, ed. F. Bolam, Technical Division, the British Paper and Board Industry Federation, Plough Place, Fetter Lane, London EC4A 1AL 1976, pp. 755-760.
59. van der Reyden, D., "Wax Pick Testing: A Preliminary Study," Art Conservation Training Programs Conference Papers, New York University, March 26-27, 1981, p. 62-73.
60. Wozniak, J.C., "The Use of Ultrasonic Measurements to Monitor the Aging of Paper", Masters Thesis, Institute of Paper Chemistry, 1985.
D. van der Reyden, Senior Paper ConservatorM. Mecklenburg, Research Engineer
M. Baker, Polymer Chemist
M. Hamill, Conservation Intern
Conservation Analytical Laboratory Museum Support Center Smithsonian Institution
Publication History
Received: Fall 1988
Paper delivered at the Book and Paper specialty group session, AIC 16th Annual Meeting, June 1-5, 1988, Vancouver, British Columbia.
Papers for the specialty group session are selected by committee, based on abstracts and there has been no further peer review. Papers are received by the compiler in the Fall following the meeting and the author is welcome to make revisions, minor or major.