FTIR Analysis of Coated Papers
by Mary Baker, Dianne van der Reyden, and Nancie RavenelThis paper was presented at the Book and Paper Specialty session of the Annual Meeting of the American Institute for Conservation of Art and Historic Artifacts, Cincinnati, June, 1989.
1 Specialty Paper Research
1.1. Introduction to the Conservation Problems of Specialty Papers
For the last several years, research into the composition of various specialty papers has been undertaken at the Conservation Analytical Laboratory (CAL) of the Smithsonian Institution. These papers include those made with specially treated pulp stock or having highly finished surfaces resulting from the application of coatings or impregnation with oils and resins. Examples of objects with these properties include drawings on papers with prepared grounds, chromolithographic prints, transparent papers, and varnished papers, such as maps. These types of papers pose special conservation problems owing to particular properties imparted by the various processing treatments.
Coated and transparent papers can absorb extraneous material, like resins and oils from tapes and other adhesives, causing embedded stains which are difficult to remove (Figure 1). The surfaces of coated papers are easily marred by scratches and abrasions which scar as well as dull or burnished the finish. Several sheets of coated papers, exposed to water, may block or stick together. Options for the conservation treatment of coated papers are limited, since use of drycleaning agents or solvents to remove stains could lead to cracking and flaking of the ground (Figures 2 and 3), or changes in the surface appearance, due to a change in color, refractive index, or reflection.
Transparent papers often discolor and embrittle upon ageing (Figure 4). Many of them are extremely hygroscopic, which renders them particularly susceptible to planar distortions. Conservation treatments need to compensate for this property. Various solvents can also cause loss of transparency.
For the purposes of the current paper, only the work done with the analysis of the coated papers will be presented.
1.2. Compositional Research
There are several treatises on the recipes for creating traditionally prepared paper . Among the oldest compositions is ground bone ash,5 but white lead, calcium carbonate, gypsum, powdered cuttlebone,6 wax, zinc oxide, titanium dioxide,9 acrylic gesso,10 clay, talc, satin white (aluminum hydroxide, calcium hydroxide and calcium sulfate), barium sulfite, and calcium sulfate, sulfite and carbonate," have also been used. These have been combined with the traditional binders such as starch, mucilage and gums (like gum arabic), gelatin and animal glues (such as hide glue), and casein, as well as modern latexes and synthetic resins (like acrylic emulsions) and peanut and soybean proteins.
There is a great deal of literature on the history and technology of coated papers. This literature covers not only the evolution of different coating compositions, but also the developing technology used to finish or apply various coatings, from burnishers to blade coaters. There is very little conservation literature on the analysis of coatings on paper.
CAL's investigation involves a review of the literature on the history, technology, and conservation problems and treatments of coated papers. From this a chronology of materials and techniques is being developed and a database of FTIR spectra of known coatings is being established to aid in the non-destructive analysis of unknowns, leading to their identification. Concurrently, paper samples with known coatings are being subjected to various solvents (water, ethanol, toluene, and acetone) applied in different ways (immersion, suction table, and poultice) in order to evaluate the effects of some conservation treatment procedures on the diverse types of coatings. Characterization of the coated papers is being undertaken before and after treatment using FTIR and SEM analysis.
The present paper outlines the procedures used for the FTIR analysis of samples of coated papers, both knowns and unknowns, and the preliminary findings. A subsequent paper will report on the effects of the various treatments.
2 FTIR Analysis
2.1. Introduction to the Use of FTIR with Museum Objects
The theory and operation of the FTIR microscope for the examination and analysis of works of art have been discussed in detail in a previous paper. Briefly, the information obtained is similar to that obtained by conventional infrared spectroscopy, the differences being that the microscope allows the instrument to "see" smaller (as small as 10 microns in diameter) samples and the fourier transform processing yields clear spectra in very little time. While the equipment is expensive, it has become a standard addition to most commercial analytical services and can be found in some museum conservation laboratories as well.
Identification by infrared spectroscopy is accomplished by either assigning chemical groups to the peaks in a spectrum and inferring the chemical formula of the sample, or by comparing the spectrum to those of known compounds and making an identification by the best match. In practice, both methods are used on each sample; the list of possibilities is narrowed by first characterizing the general chemistry of the sample (such as "protein", "oil", "polysaccharide") and then comparing the spectrum with known spectra from that general classification. Often, an exact identification of a museum sample can not be made, due to the similarity of spectra obtained from different compounds of the same chemical class, but the general classification can still aid the conservator. For example, while it is rarely possible to differentiate between the various gums by FTIR, the characterization of the unknown as a gum is often sufficient to help the conservator with treatment decisions.
Figure 1. Ink drawing on coated paper with embedded stains from pressure sensitive tape.
Figure 2. SEM photograph showing cracking of a BaS04/CaC03/acrylic coated sample treated with aqueous poultice.
Figure 3. SEM photograph showing loss of burnished surface of a ZnO/hide glue coated sample treated by acetone immersion.
Figure 4: Transparent watermark paper showing discoloration and embrittlement.
2.2 Traditional and New Methods
All FTIR analysis is technically "non-destructive" since the procedure does not alter the sample, allowing it to be used for subsequent analysis. However, in a conservation context, the very removal of that sample from the object for analysis could be considered "destructive". Fortunately, many objects can be analyzed by FTIR in a truly non-destructive method requiring no sampling; this involves using the FTIR in reflectance mode and obtaining the spectrum from bouncing the IR beam off the surface of the object. Unfortunately, microsampling is still necessary if analysis is to be done beneath the surface or when the reflected signals are not clear enough.
Because the FTIR microscope can analyze such small samples, a method was developed of removing a single fiber from the coated paper, pressing it flat between two halves of a diamond sample holder, and analyzing the results by transmission of the IR beam through the sample. The method provided two distinct regions to analyze: the flattened fiber and the relatively pure coating material on either side 9f it. Additionally, a microtoming technique described by Jia-sun Tsang was employed to look at the depth of penetration of some of the coatings by transmission FTIR.
Presented here are examples of both reflectance and transmission spectra of the pigments and binders from several coated paper samples, chosen from the many samples of gated paper which have been characterized with the FTIR microscope at CAL.
2.3 Results
2.3.1 Reflectance
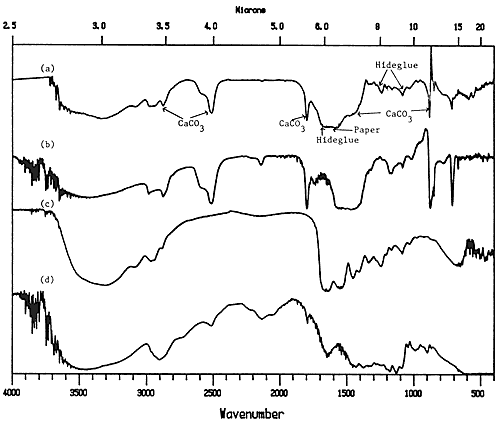
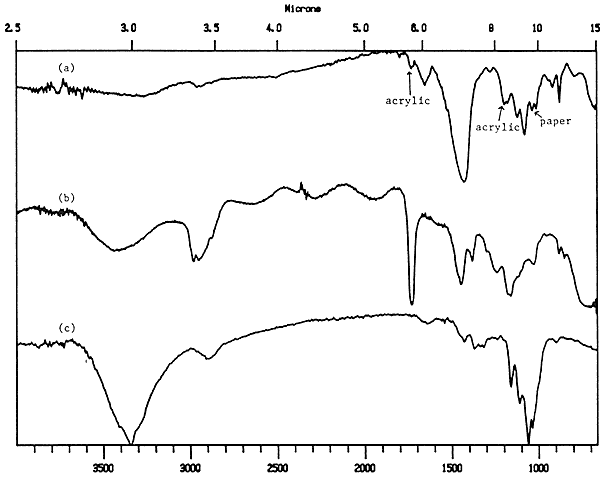
Infrared beams can penetrate all but the thickest of coatings, so that a reflectance spectrum of a coated paper is usually a composite spectrum of the substrate (paper) and the coating. Therefore, if the coating medium or pigment is to be identified, its spectrum must be different from that of the paper. Gum arabic could not be identified by this method, because the spectra of gums and cellulose are nearly identical. Proteins, however, such as hide glue and casein showed up well. As shown in Figure 5, the peaks at 1550 cm-1 and 1656 cm-1 ,indicative of proteins, are superimposed on the cellulose and pigment peaks, but the 1650 cm-1 absorbance can still be distinguished, although it is very close to the cellulose peak at 1635 cm-1. It is important to have good reference spectra, taken with the same instrument used for the samples, so that such small differences in peak location can be seen.
Upon close inspection, the small peaks between 1300 and 1000 cm-1 can be seen to match between the coated paper sample and the hide glue reference. Since the chemical compositions of the different proteins, such as hide glue, casein and albumin, are different, one should be able to distinguish between their spectra. In practice, it is more difficult than it sounds, but some attempts have been made . However, the general characterization of "protein" is easily made from the reflectance spectrum.
Figure 5. Reflectance spectra of a) CaCO3/hide glue coated paper; b) CaCO3 reference; c) hide glue reference; d) plain paper reference.
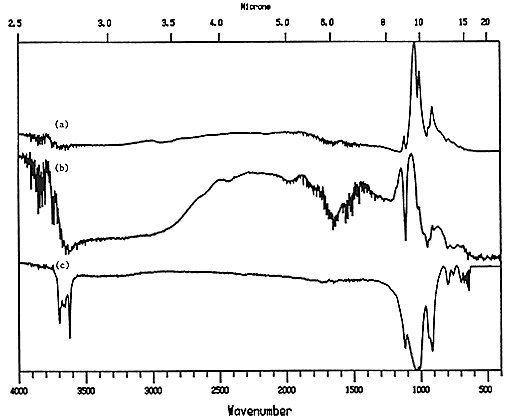
Figure 6. Reflectance spectra of a) coated magazine cover and b) kaolin reference; c) transmission spectrum of kaolin.
Acrylic emulsions also have spectra which should show up well against cellulose, but an acrylic coated paper could not be successfully identified by the reflectance method. It could be seen that the acrylic emulsion made a much thinner coating than the other binders; the thin layer did not produce a strong enough spectrum to be seen against the very strong paper spectrum.
Pigments, such as calcium carbonate, barium sulfate and kaolin, show up clearly with this method. The spectrum of calcium carbonate in Figure 5 contain many strong peaks (2550, 1800, 1500-1300, and 890 cm-1) which show up in the spectrum of the coated paper. The strangely shaped peak at 890 cm-1 is often found at various wavelengths in reflection spectra. It is due to a reflectance phenomenon on the surface which occurs certain wavelength (called Restrahlen bands) which are particular to the compound.
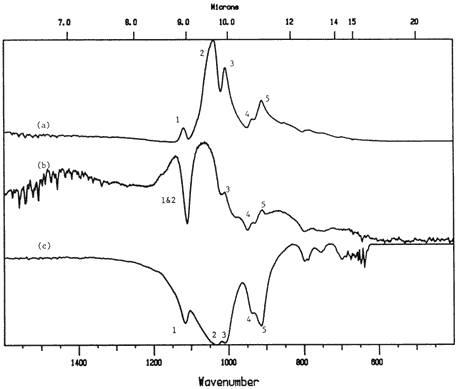
Kaolin, often found in coated book or magazine stock, showed up clearly in a coated comic book cover (Figure 6), although the spectral anomaly (due to a Restrahlen band) which showed up in the reference reflection spectrum of kaolin (around 1160 cm-1) makes the comparison difficult; in this case it was easier to compare the sample to a transmission spectrum of kaolin, which looks almost like a mirror image of the reflectance spectrum from the sample. The area between 1600 cm-1 and 400 cm-1 is enlarged in Figure 7 to show the similarity between the spectra, which is not obvious at first glance. The two reflectance spectra and the transmission spectrum all have peaks 3, 4 and 5 (which are "up" peaks in the reflectance mode and "down" peaks in the transmission mode). The coating spectrum and the transmission reference spectrum also share peaks 1 and 2 in the same way. The reflectance reference spectrum does not show peaks 1 and 2 due to the large Restrahlen band-caused spectral anomaly that occurs in the same location as peaks 1 and 2. The fact that the anomaly appears in the reference spectrum but not the sample spectrum suggests a slight difference in chemistry between the two, such as a difference in the degree of hydration of the two kaolin samples, or a difference in surface texture.
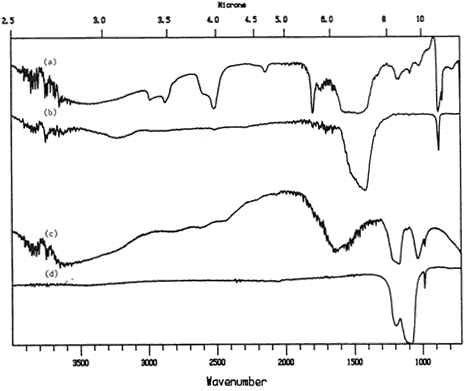
Reflection spectra are almost always different from transmission spectra, due to Restrahlen bands and changes of peak intensities between the different modes. In Figure 8, the reflectance spectrum of calcium carbonate appears to have more peaks than the transmission spectrum due to the weakness of those peaks in the transmission mode. For example, the peak at 2550 cm-1, which is so strong in the reflectance spectrum, is barely noticeable in the transmission spectrum. In the same figure, the reflectance spectrum of barium sulfate is slightly different from the transmission spectrum. It is important, therefore to use reflectance spectra as references for other reflectance spectra and likewise for transmission spectra. Additionally, in reflectance work, changes in the spectra caused by slight chemical differences between the sample and the reference must be considered and accounted for.
2.3.2 Transmission
The method of removing a coated fiber from the paper and pressing it flat provided good spectra for the protein and acrylic samples. Figure 9 shows the transmission spectrum of an acrylic coated paper fiber (the acrylic is identified by the peaks at 1725 cm-1 and 1200 cm-1). This was obtained by collecting the spectrum at the very edge of the flattened fiber, where the acrylic was most concentrated and the interference from the paper was minimized. The pigment appears to be calcium carbonate, as it matched well with the reference spectrum. It was still difficult to identify gum arabic, as the spectra of the gum are still too similar to that of the paper.
Figure 7. Reflectance spectra of a) coated magazine cover and b) kaolin reference; c) transmission spectrum of kaolin (enlarged to show detail between 1600 cm-1 and 400 cm-1).
Figure 8. Spectra of CaCO3 a) reflectance and b) transmission; spectra of BaSO4 c) reflectance and d) transmission.
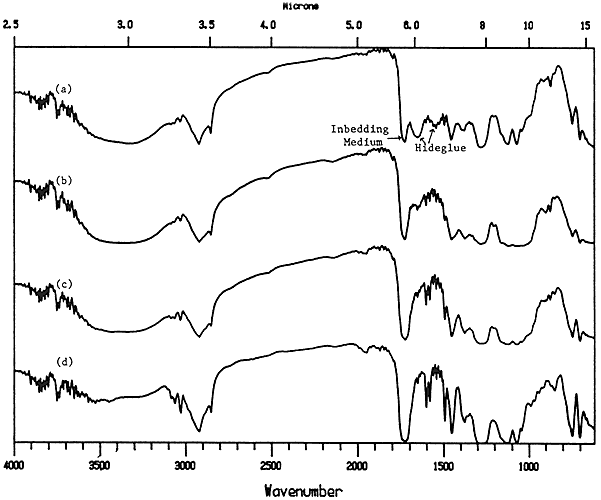
The depth of penetration of hide glue into paper was successfully measured using a microtomed cross-section of the coated paper. Figure 10 shows the spectra of different areas of the cross-section, the top spectrum being near the surface and the bottom spectrum being about 50 microns from the surface. The protein peaks (1650 and 1550 cm) slowly disappear as the distance from the surface increases. While this method would not usually be employed on an actual object, it looks promising for our studies into the effect of solvent treatments on prepared samples of coated papers.
3 Conclusions
Certain binders and pigments can be identified without sampling by the reflectance method using the FTIR microscope. Other binders can only be identified by transmission, which requires sampling, but the sample can be as small as one fiber, due to the power of the instrument. The analysis of cross-sections of the coated papers looks promising for research on the effects of treatments on the coated papers. Overall, the FTIR microscope proves to be a useful instrument for identification of and research on specialty papers.
Figure 9. Transmission spectra of a) single fiber from commercially prepared coated paper; b) Liquitex acrylic medium reference; c) plain paper reference.
Figure 10. Transmission spectra of cross-section from hide glue coated paper a) surface layer; b) about 15 microns from surface; c) about 30 microns from surface; d) about 50 microns from surface
Notes
1. In one of the few publications referring to deterioration of coated papers, Margaret H. Ellis, in "Metalpoint Drawings: Special Conservation Problems for Collectors", Drawings, Vol. 2, No. 3, Sept.-Oct. 1980, pp. 59-61, notes problems of abrasion, flaking, fingerprint and oil stains, water damage and stains, and foxing and mold growth on coated papers, and discusses their causes and preventions.
2. Very little has been published referring to deterioration of coated papers. Ellis, Op. Cit., p. 60, notes that water can dissolve some grounds, causing dull areas or tide lines.
Eric Harding in "Restoration of Drawings executed on Prepared Paper", Conservator, Vol. 7, 1983, pp. 24-28, outlines treatments using steam on the verso of a secondary support to remove it from a prepared ground paper, and using pastels to obscure a disfiguring dark spot on another ground which had resulted from a 19th century repair. He writes that treatment of foxing with aqueous solutions applied with small sable brushes could disrupt surface reflective properties.
Paula Volent, in "The Conservation Treatment of an Oversize Drawing by Ann McCoy, The WAAC Newsletter, Vol. 10, No. 3, September, 1988, pp. 6-8, writes of removing masking tapes from the reverse of a photographic backdrop prepared with chalky acrylic gesso using heat, while adhesive residues were softened by heptane and mechanically removed. Tears and breaks were repaired with Japanese tissue and wheat starch paste, reinforced with acrylic gesso.
Unpublished preliminary data collected at CAL has indicated that cracking of coated papers, visible with SEM (Figs. 2 and 3), may indeed be induced by water (applied as poultice in diatomaceous earth on a modern commercially prepared paper having a barium sulfide/calcium hydroxide/acrylic binder coating) or by acetone (following immersion of a sample paper prepared with zinc oxide and Liquitex gel acrylic). This work was done as part of a project which involves subjecting several types of coated and transparent papers to treatment with four solvents (water, ethanol, toluene and acetone) applied in three ways (immersion, suction table, and diatomaceous earth poultice). The findings, along with a technical history of coated and transparent papers, will be published by CAL staff in the near future.
3. For instance, additional unpublished research at CAL has indicated that acetone, applied as a poultice or using a suction disk, can cause loss of transparency of the commercially prepared test paper (Albanene Prepared Tracing Paper, Keuffel and Esser Co., Persippany, N.J. #105353, which is advertised as 100% rag, but which FTIR analysis indicated might have some plastic or oil treatment, judging by peaks at 1760 and 1770). Further details of this study will be published, as noted above.
4. 0ne of the oldest treatises on prepared paper, which was used primarily in the 14th-16th centuries, before the invention of pencils or drawing materials such as conte crayons, is C. Cennini's 14th century work, The Craftman's Handbook, Dover, 1933, translated by D.V. Thompson. Other sources of recipes are Watrous, J. The Craft of Old Master Drawings, University of Wisconsin Press, 1957; Meder, J., and W. Ames, The Mastery of Drawing, Arabis Books, New York, 1978; and Wehlte, Kurt, Materials and Techniques of Painting, Van Nostrand Reinhold, New York, 1982.
7. Pan Jixing, in "Ten Kinds of Modified Paper of Ancient China: A Summary", Institute of Paper Historians Information, Vol. 17, No. 4, 1983, page 152, notes the use in ancient China of wax mixed with white mineral powders to produce coated papers.
11. See, for instance, Clarence J. West's "The Institute of Paper Chemistry Bibliographic Series No. 162: Coating of Printing Papers", Appleton, Wisconsin, 1952, which covers primarily developments in coating technology to improve printing techniques.
12. Combinations of these materials are used as well. For instance FTIR analysis of a commercially produced modern paper found a combination of barium sulfate and calcium carbonate.
15. For instance, for a synopsis of developments up to 1940, see Maclead, Martin, "Early History of Coated Papers-How They Came of Age," Paper Trade Journal, May 27, 1972, pp. 170-175.
16. Harding, Op. Cit., p. 27, mentions using X-ray fluorescence to detect lead in a Botticelli study. Ellis, Op. Cit., p. 6, refers to analysis of metals for metal point drawings, using XRF and neutron activation energy.
17. Baker, M., D. von Endt, W. Hopwood and D. Erhardt, "FTIR-microspectrometry: A Powerful Conservation Analysis Tool," American Institute for Conservation of Historic and Artistic Works. Abstracts of Papers Presented at the Sixteenth Annual Meeting, pp. 1-13, New Orleans, June 1-5, 1988.
18. Information about labs using these instruments can be obtained by calling the manufacturers (Spectra-Tech: 203-357-7055, Analect: 714-660-9269, and Bio-Rad, Digilab Div.: 617-868-4330); the sales departments keep user lists arranged by location.
19. Tsang, J.-S., and R. H. Cunningham, "Some Improvements in the Study of Cross-Sections," The American Institute for Conservation of Historic and Artistic Works. Abstracts of Papers Presented at the Seventeenth Annual Meeting, pp. 20-21, Cincinnati OH, May 31-June 4, 1989.
20. The examples chosen for this publication are: 1. A laboratory prepared sample using a mixture of calcium carbonate (Baker Reagent grade, #1294) and rabbit skin glue (Liquitex Glue Ground #4450) applied to paper; 2. The cover of a soft cover publication (Marvel comics) found to have a coating of kaolin and an acrylic; 3. A commercially available coated paper (Cornellisan), which was found to have a coating of barium sulfate, calcium carbonate and acrylic. The spectra were collected on a Mattson Cygnus 100 FTIR with a Spectra-Tech IR-Plan Microscope attachment.
21. Perron, J., "The Use of FTIR in the Study of Photographic Materials," Topics in Photographic Preservation, Vol. 3, 1989, pp. 112-122.
22. Restrahlen bands yield "spectral anomalies", which are usually characterized by a sudden rise in the spectrum above the baseline, followed by a sharp dip, usually of equal magnitude as the rise, giving the "s" or "z" shape to the peak. Often, the dip is small, giving the appearance of an "upside-down" peak above the baseline. When there are several of these bands together, it is difficult to distinguish the spectral anomalies from "normal" peaks. Since the bands are the result of a surface phenomenon, the spectra will change if the surface texture of the sample is changed; this property can be used to distinguish the bands, as the normal peaks should not change.
Mary Baker, Research ChemistDianne van der Reyden, Senior Paper Conservator
Nancie Ravenel, Conservation Intern
Conservation Analytical Laboratory, Museum Support Center
Smithsonian Institution
Publication History
Received: Fall 1989
Paper delivered at the Book and Paper specialty group session, AIC 17th Annual Meeting, May 31-June 4, 1989, Cincinnati, Ohio.
Papers for the specialty group session are selected by committee, based on abstracts and there has been no further peer review. Papers are received by the compiler in the Fall following the meeting and the author is welcome to make revisions, minor or major.