In July the Institute for Standards Research held a workshop at ASTM headquarters in Philadelphia to plan a research program on paper permanence. Sixteen papers were given on the first day, and structured discussions filled the second and third day, leading to a set of recommendations. After the meeting, these recommendations were summarized by a group of four participants and later worked up at ISR into a request for proposals, itself in the form of a research proposal, which was mailed out on or before November 7. A summary of that request for proposals is included in this issue. A number of proposals were received, and no more are being invited. The next steps will be to identify funding and select a research contractor.
In the meantime, those workshop papers should not be forgotten. The last time the papermaking world and the cultural world came together to talk about permanence was six years ago, when the TAPPI Paper Preservation Symposium was held in Washington, D.C. The next time may be many years hence. The ISR papers have been published, and are available for $95 + S & H from either NISO Press (800/282-NISO) or ASTM Customer Service (215/299-5585, fax 215/977-9679). The papers that seemed to have the most potential for facilitating or indicating the direction of future research will be summarized below.
Terry Norris, retired V.P. of Research & Development, Great Northern Nekoosa Corp., led off with his paper, "Over-view of Paper Deterioration," in which he credited the paper industry for solving the paper shortages of the 1800s, although it solved them by methods that led to today's problems. He also credited those who have investigated the mechanism of paper deterioration, and said he believed there now existed a good knowledge base concerning the cause and the mechanisms of paper deterioration, partly because the printers and publishers, libraries, archives and museums have made their voices heard.
Norris describes the structure of the paper fiber, the relationships of cellulose, hemicelluloses and lignin in it, and their nature and vulnerability to degradative influences, which he lists. On the questions of whether high-yield pulps can be used in permanent papers, and whether the research planned can provide an answer, he warns that "the composite media nature of paper makes it difficult to achieve verification, validation and confirmation of research results. . . . We are limited in our use of theory to plan experiments or to predict the outcome of the next experiment. This means that we must use a heuristic approach in our efforts to decide what is ultimately possible scientifically. . . ."
Paul Banks, senior lecturer in the Preservation and Conservation Studies program at the University of Texas, spoke on "Paper Permanence: Present Needs and Future Possibilities," saying that regardless of the role that digitization plays now or in the future, there will always be an immense number of paper books, documents and works of art that must be preserved in their original format, because there will never be enough money to digitize all of them. All methods of copying and treatment, including deacidification, are expensive, and at the same time, there are new demands on shrinking budgets in libraries and archives.
Control of the environment in which collections are stored calls for an initial investment in environmental control systems, even sometimes a new building or addition, but it is economical because it makes possible the preservation of entire collections. It could be even more economical, he said, if aging research could provide collecting institutions with "persuasive and quantitative evidence of the benefits that they will earn from different levels of improved environment." Lack of such evidence in the past has held up development of environmental standards for storage of paper records.
As an example of the type of situation in which guidance on costs and benefits is needed for decision-making, he posited a collection in a particular climate where "the difference between specifying 35% relative humidity and 50% relative humidity might be the necessity of installing an entire secondary dehumidification system, with attendant large initial and operating costs." Similar choices might be necessary for temperature, and especially for pollution control.
He discussed standards briefly, saying that the underlying issue of the workshop was manufacturing specifications versus performance standards. But, he said, "Versus is putting it too strongly; all of the current paper permanence standards contain both kinds of provisions, and I believe that most people involved would not advocate a standard that was strictly one or the other." The standard's limitation of lignin and requirement of a 2% alkaline reserve are manufacturing specifications, while the minimum tear resistance is a performance specification.
Regarding a requirement for accelerated aging [which the paper industry generally prefers because it is a performance rather than a manufacturing specification], he saw two large challenges to its use in permanence standards: identifying and agreeing upon the aging procedure, and the difficulties of monitoring compliance. The second challenge is the most serious one, because aging procedures are so costly that few libraries, archives or publishers can afford to use them to verify compliance. They would also be costly for mills.
Paul Whitmore, Director of the Research Center on the Materials of the Artist and Conservator at Carnegie Mellon University, spoke on "The Mechanisms of Chemical Deterioration of Paper," focusing on those reactions which over time will alter physical properties, rather than appearance.
First he described the most important aspect of cellulose degradation, the scission of the chains which provide strength to the paper. He went on to explain that the degree of polymerization (DP) of the cellulose, while a good measure of the length of cellulose chains, is not a direct measure of the scissions of those chains; instead, the reciprocal of the DP reflects the scissions. As a result, a given number of scissions will lower the DP of a high-DP paper dramatically while having a smaller effect on a low-DP paper. To compare the degradation of two different papers, then, one must track the scissions as 1/DP changes, rather than DP losses.
Another point that was made was the relationship between cellulose chain scission and paper strength loss. In strong paper, where many cellulose chains contribute to strength, it is likely that breaking any chain will decrease the strength of the paper. As the paper becomes weaker, and few intact cellulose chains remain, it becomes clear that further scissions are more likely to occur on already broken chains, and thus will not affect the strength. This helps to explain why books do not literally crumble to dust, but linger indefinitely in a terminal state of brittleness.
Chain scission is usually monitored by changes of viscosity of cellulose solutions or gel permeation chromatography. These are the best way, though they are not ideal. Other methods use alkali solubility, copper number, or alpha cellulose content. No method works very well in the last stages of degradation.
Cellulose chains are broken mainly by hydrolysis, oxidation, and elimination (peeling). It is hard to tell whether hydrolysis or oxidation has caused scission in a given sample of cellulose.
Oxidation is a rather slow process in pure cellulose, unless oxidizing agents or alkaline catalysts are present. Agents such as light or chemical bleaches can initiate it.
Elimination reactions in normal cellulose occur next to carbonyl groups, which are located at the reducing end of the chain. They do not usually reduce the DP unless the cellulose has been oxidized. This forms carbonyl groups along the chain as well as at the end, and each carbonyl group is a point of vulnerability to scission. Oxidation and elimination thus provide an example of synergy. Oxidation and hydrolysis are synergistic too. The chemical reactions can get complicated; but, Whitmore says, the only significant interactions are those that affect the cellulose scission chemistries, and in the early stages of degradation, only a few chemistries may dominate.
Basic reactions in paper chemistry are well understood; the issues on which research is still needed include a good way of finding the molecular weight distribution of lignin-containing papers, so that DP can be calculated. We also need to know which cellulose scission chemistries dominate the aging process of papers of specific composition, which are aged in specific environments (including in the aging oven); and we need to know about other processes that may affect those cellulose scission chemistries (including the effect of lignin, gaseous pollutants and alkaline reserve).
Derek H. Page, Distinguished Professor of Physics at the Institute of Paper Science and Technology, spoke on "The Structure and Strength of Paper." Strength [of new paper] is "directly and precisely proportional to the cellulose content," he said, as long as the wood is pulped by methods that do not degrade the cellulose.
DP is not a measure of cellulose strength, he said, because the fiber can be weakened, as by sulphite pulping, but not actually broken. Conversely, the vapor phase reactions typical of natural aging will lower DP without affecting strength, because the reactions are homogeneous.
In research he has conducted recently, his results indicate that both natural and accelerated aging degrade fiber strength but have no effect on bond strength. The only important reaction in strength loss is acid hydrolysis. Oxidation is not a primary mechanism because the presence of oxygen during aging has no effect on the degradation. (He does not mention the effect of catalysts or oxidizing agents.) He is surprised that more controlled experiments, like those recently done at Paprican, have not been carried out relating the rate of degradation to initial pH.
In his "Conclusions" section, he advocates use of "a more fundamental strength test such as zero span rather than a more arbitrary test such as folding endurance" for measuring loss of strength in aging paper; and he says that "maintenance of neutral and alkaline conditions in the sheet are sufficient to retain strength upon natural or accelerated aging."
Rajai H. Atalla, Head of Chemistry and Pulping Research at the U.S. Forest Products Laboratory in Madison, spoke on "The Mechanisms of Optical Deterioration of Paper." In paper, he said, there are three main classes of components that absorb light: 1) cellulose and the hemicelluloses (mainly the hemicelluloses) that include functional groups with doubly bonded atoms, 2) the constituents of lignin, a phenyl propanoid polymer with many different functionalities, and 3) transition metal ions, which occur in all lignocellulosic matter, most commonly manganese, iron and copper, and sometimes cobalt and nickel. Though these occur in relatively small quantities, he said, they are important because they can catalyze the action of photo-excited electrons. They can come from the wood itself, perhaps more from high-yield pulps; from the process waters; or from mill equipment.
Two other classes of components are extractives (soluble substances not part of the cell wall) and proteinaceous matter. He said that the extractives may require special attention in the future if their complexes with transition metal ions are shown to be important to color stability. In some contexts, the role of proteinaceous matter may also need to be looked into, not because it can initiate any unusual photochemical processes, but because it is a neglected area of research.
Although papers from chemical pulps do yellow in response to light, much more research has been done on the response of papers made from mechanical pulps. By far the most significant class of reactions, he said, are the photochemically initiated oxidation reactions, and their primary impact is on substructures of lignin, which may undergo either photo-bleaching or darkening, depending on circumstances. It gets even more complicated: these pulps usually retain more extractives and transition metal ions, and the role of metal ions has not received its share of research in this area.
It is hard to use accelerated testing (aging) for optical properties, because elevated temperatures do not simulate the reactions under consideration, but dramatically increase the thermal quenching of photo-excited electrons. Simply using higher photon fluxes (brighter lights) risks activating reaction pathways that are not normally accessible and masking the paths that are relevant.
Gordon Leary (Executive Director, Mechanical Wood-Pulps Network of Centres of Excellence) and Xuejun Zou (Scientist, Pulp and Paper Research Institute of Canada) had a paper titled, "The Optical Properties of Lignin-Containing Papers as They Relate to Paper Permanence." They define permanence to include ability to withstand handling after aging, as well as legibility and ability to give good photocopies. There is no fundamental reason for aging-related brightness loss to be accompanied by loss of strength, they say. Chemical changes do not necessarily affect both qualities to the same degree; although acid hydrolysis both darkens and weakens paper, ozone can improve the strength of lignin-containing papers at the same time it darkens them, because it forms new, stronger inter-fiber bonds by reacting with lignin.
The mechanism of light-induced yellowing is complex, taking place in five stages: light absorption by lignin chromophores; formation of mobile radicals; formation of lignin radicals; formation of yellow products via breakdown or oxidation of the lignin radicals by oxygen; and recycling of some products as chromophores. The authors believe that the lignin protects the cellulose from radical-induced degradation and functions in much the same way as an antioxidant. It is their understanding that light-yellowing predominately breaks down the lignin and occurs quite independently of the carbohydrates.
Yellowing could be stopped by preventing the absorption of UV light, preventing the initial formation of radicals, or by preventing the oxidation of phenoxy radicals to quinones. Research in the Mechanical Pulps Network has had some success, but the ultimate challenge will be to find a treatment that is both effective and economical to apply.
To check whether yellowed newspapers were still legible and photocopyable (part of their definition of permanent), they gave a 1994 newspaper a 24-hour exposure to light that approximated 5000 hours under office lighting. This reduced its brightness from 56% to 34%. By adjusting the photocopier, they were able to get a clear copy. They concluded that legibility was not a problem.
To investigate the relationship between strength loss and brightness loss with light-aging, they compared some bleached CTMP and bleached kraft irradiated with UV for 113 hours at 60°C and 50% RH. Although both had pHs around 6.0, their initial strength differed: 216 MIT double folds for the CTMP and 3535 for the bleached kraft. A graph, reproduced here, shows that the two papers also reacted differently to the light aging: the CTMP brightness fell to 30% of its initial value, and strength to 35%, or about 76 double folds. The brightness of the kraft pulp paper declined hardly at all (from about 84 to about 81), but its strength fell to 47% of its initial value. These results are presented to show that light-induced yellowing, at least initially, has little effect on strength of lignin-containing paper.
UV-irradiated paper sheets (@ 60° & 50% RH)
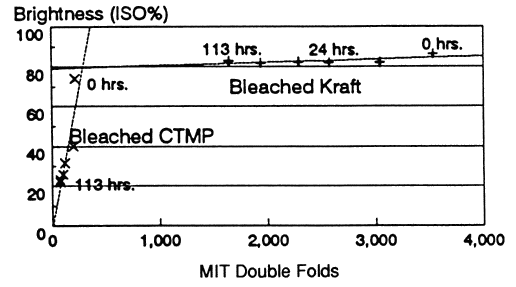
Figure 5. Variation of Brightness and Strength of Paper
Sheets made from CTMP or Kraft pulp and irradiated with light for
different times.
One of the conclusions in the final section is that neither light- nor nitrogen dioxide-induced yellowing of lignin-containing papers is accompanied by loss of strength.
Chandru J. Shahani, Preservation Research Officer at the Library of Congress (LC), spoke on "Accelerated Aging of Paper: Can it Really Foretell the Permanence of Paper?" In his paper he gives arguments for and against the use of accelerated aging, reviewing the work and conclusions of three skeptics who have published in Restaurator (Copenhagen): Helmut Bansa, H.-H. Hofer and E. Ströfer-Hua.
Nevertheless, he says, there is no substitute for it in the laboratory, if one wants to do research on the permanence of paper. Results must be interpreted with caution, but accelerated aging is a good tool.
Three sets of conditions are favored in labs today: 80°C/ 65% RH, 90°C/50% RH, and 90°C/25% RH. (Here he mistakenly credits ISO for incorporating an accelerated aging test into its new paper permanence standard; actually, ISO merely explained why it did not include accelerated aging as one of its requirements.)
Research on accelerated aging is performed at the Library of Congress, the Swedish Institute of Pulp and Paper Research, the Conservation Analytical Laboratory of the Smithsonian Institution, the Canadian Conservation Institute in Ottawa, and the Carnegie Mellon Institute in Pittsburgh.
His own research has dealt with the effect of enclosure on the rate of degradation of paper in the aging oven. This included the effect of aging when paper is stacked or piled, instead of hanging freely in the oven. He found that the piled-up and enclosed papers aged much faster than freehanging paper, and were more acidic by a half a pH unit. They also aged at an increasing rate, possibly because of the accumulation of degradation products within the paper, whereas the single sheets aged at a steady rate.
An interesting finding was that the paper aged in stacks smelled like old books do when they are opened.
In a related study, the effect of sealing paper in clear polyester enclosures was very like that found for the paper aged in piles. The paper became brittle by the tenth day of aging, unless it was first deacidified. Embrittlement did not occur, however, if the paper contained an alkaline reserve. The presence of an alkaline buffered paper in the same enclosure gave some protection to acidic papers.
Of the two patterns of aging exemplified by the single sheets and the stacks of paper in the oven, he believes that the stacks, with their increasing rate of degradation, rather than the single sheets, are typical of natural aging,. The inference is that brittle acidic paper will not last indefinitely if it is not handled. He calls for a broad consensus on which properties to test in order to monitor the aging process. He also puts in a good word for the fold endurance test. The Library of Congress had just completed a lengthy study comparing a large number of chemical and physical properties in three different papers aged for three months at 90°C and 50% RH. His paper presents data on fold endurance, tensile energy absorption, tear and burst for one of the papers. Fold endurance is of course most sensitive; it also has the least variation, with all the data points lying neatly on the curve, while data points for the other measures straggle somewhat.
Norayr Gurnagul (Scientist at Paprican) gave a paper on the "Canadian Cooperative Permanent Paper Research Program: Development Process: Physical Testing." He described a joint industry/government research initiative at Paprican and the Canadian Conservation Institute (CCI), in concert with the Canadian General Standards Board, to examine the impact of lignin on paper permanence. No results are reported here, but the thinking that went into the research plan is described. They decided to allow but not require the folding endurance test, for monitoring the effects of accelerated aging. Brightness will be measured, along with tear strength, tensile strength, sheet stretch and TEA. A recently introduced brittleness index will be calculated from the stress/strain curves, which are computed from the TEA and the elastic strain energy at the time of failure.
A paper by Helen Burgess and four co-authors from the Canadian Conservation Institute was read by David Grattan, describing the chemical testing aspects of the same joint research program. The CCI will focus on the resistance of high yield pulps to chemical changes, and the effects of air pollution on papers made from these pulps. Both handsheets and commercial paper samples will be studied. Tests to be used include Klason lignin (except with the bleached kraft pulps), determination of metal ions, cold extract pH and also cold extract pH in 0.1M NaCl by the Scallan method (to release acid from within the fiber wall and give a more realistic pH reading), alkaline reserve content and DP. They may also test for hot alkali solubility (an early indicator of chemical degradation) and carbonyl content. One problem they still have to solve is finding the DP of pulps with high lignin content.
John Havermans (Workgroup Leader and Conservation and Research Scientist at the TNO Centre for Paper and Board Research in Delft) gave two long papers on a three-year research project, funded partly by the European Community, to discover the effect of polluted air on papers of all sorts. Both papers must be considered as drafts, because the final report was not scheduled for publication until the end of 1994. Quoted selections and passages have been edited slightly to make them read more smoothly.
1. "STEP CT 90-0100, The Effects of Air Pollutants on Accelerated Aging of Cellulose Containing Materials: Paper Preliminary Results." (21 refs)
2. "Main Conclusions of the STEP CT 90-0100 Project: Influences of Air Pollutants on the Accelerated Aging of Cellulose-Based Materials: Paper." (An update, written June 1994)
1. Preliminary Results. The project, carried out in three countries, used new and old papers as well as handsheets; naturally and artificially aged archive papers, books, newspapers and maps; acid, alkaline, lignin-containing and chemical pulp papers. Papers were aged, exposed to air pollutants (sulphur and nitrogen dioxide in the ratio 1:2), and deacidified, not necessarily in that order. The three deacidification processes chosen for testing were diethyl zinc (DEZ); methyl magnesium carbonate as used in Sablé, France; and magnesium butoxytriglycolate, which is the Lithco process. They were used on selected old materials and acid materials.
In his review of changes in paper that take place with aging, Havermans described some of the conditions under which hydrolysis may take place, saying, "Traces of metals, such as iron and copper together with SO2 or NO2 promote the hydrolysis of cellulose. Cellulose may also be hydrolyzed in an alkaline environment. . . . Under the influence of oxygen or light, lignin may degrade more rapidly than cellulose, and monomeric compounds may be formed. In an alkaline environment the important links in lignin may be hydrolyzed. The resulting acid monomers may promote cellulose hydrolysis." [It is unusual to hear that metals and alkaline conditions can bring about hydrolysis.]
They carefully examined the published studies of indoor pollutant levels and relative concentrations of SO2 and NO2, when they were deciding on realistic levels and proportions to use for this study.
Aging was done at 90°C and 50% RH. Samples were exposed to pollutants in specially-built "exposure chambers" of glass and teflon (Sweden) or plexiglass (Netherlands and France). All three deacidification methods were performed by the labs themselves, apparently, and all three were found to give uneven results: uneven deposition within the books, between the methods, and for the different kinds of paper: the pH varied between 7 and 10. [Other studies of deacidification methods have found similar results.]
The reactions of the papers to pollutants were interesting. Some papers (sulphite, linters and acid copy papers) stopped taking up SO2 after 10 hours of exposure, as if they had reached the limit of their capacity. The papers containing calcium carbonate or lignin absorbed the most SO2, and ground CaCO3 took up more SO2 than the precipitated did. Acidic papers tended to take up less SO2. All papers took up more SO2 when it was used with NO2. Increasing the RH caused an enormous increase in the deposition rate.
Papers exposed to pollutant gases showed a decrease in DP, and an increase in alkaline extractable material and the copper number. Their strength and pH dropped dramatically. Infrared spectroscopy showed an increase in crystallinity. The copper number (an indicator of oxidation) was two to five times as high for two acidic papers (sulphite and cotton) as for comparable alkaline papers. Zero-span (an indicator of fiber strength) fell after exposure for all the papers, but it fell much further, predictably, for the acidic papers.
The groundwood papers picked up more pollutants than the acid woodfree papers, but they deteriorated at about the same rate.
2. Main Conclusions. This paper presents almost 100 conclusions, remarks and recommendations resulting from the project. Thirty of the conclusions relate to the deposition of SO2 and its reactions with all the papers. They are grouped by factor or topic, e.g., "Naturally Aged (Old) Papers," or "Effect of Groundwood."
There are 17 more conclusions from the pollution and aging experiments, and 20 conclusions on deacidification, similarly grouped. Following all the conclusions are five remarks, nine recommendations relating to the care of paper collections, and 16 recommendations for future research, both general and specific.
This is an immense study. It will provide food for thought for many years to come. By the same token, it is hard to summarize. Here are some of the more interesting conclusions and recommendations that have not already been summarized:
The recommendations for preservation of paper are fairly consistent with accepted practice: to deacidify acidic papers; to store books and papers at 40-50% RH; and, in buildings where pollutants are not removed from the air, to store paper in alkaline enclosures or enclosures made with "specific absorbers" (activated carbon, perhaps, or molecular sieves).
The recommendations for future research are omitted from this summary, because they are of less general interest, although they are important. It is assumed that everyone in a position to follow up on these recommendations will have a complete copy of the research report.
Tom Lindström, Director of Research at MoDo Research & Development, Sweden, described the Swedish R & D Project on Paper Preservation, whose activities resemble those of a national preservation program: building up expertise, evaluating available treatment methods, surveying the condition of books in libraries, promoting use of long lasting printing and writing paper grades, and evaluating the aging of paper. The project is carried out cooperatively by major institutions. Ten publications and research reports are outlined. Their titles and major findings are listed here, as given in the overhead transparencies from which Lindström spoke. Copies of these reports may be obtained from Ingmar Fröjd, Project Coordinator, National Preservation Project, National Archives, Box 12541, S-10229 Stockholm, Sweden (fax 46-8-737 6474).
Among the suggested topics for further research are 1) the interplay of NOx/SO2/environmental conditions, and 2) chemiluminescence in the low temperature region. Regarding mechanical pulps and paper permanence, some conclusions are:
1) Heat induced aging may not be the issue (strength, brightness). More systematic data on alkaline buffered systems are required though.
2) Light induced aging is, however, critical. Influence of test conditions (light intensity, wave length spectrum, temperature, humidity) need to be better understood.