

Revised and reprinted from the Abbey Newsletter, August 1987, v.11 #6, p. 87-89. The letter from Dr. Rulfs is reprinted from p. 125 of the December 1987 issue. Dr. Rulfs, who retired from the University of Michigan Chemistry Department a few years ago, is the author of a number of articles in Analytical Chemistry and the Journal of the American Chemical Society, and of three chapters in Kolthoff and Elving's Treatise on Analytical Chemistry.
Four years ago, the Editor took part in a study of the pH of newly acquired books at Columbia University (Abbey Newsletter, Feb. 1984, v.1 #1 pt.1, p.2). Part of that
study involved a test for the presence of alkaline reserve (calcium carbonate) in the book paper. A tiny sample was moistened with a drop of acid (6N HC1) and observed under a microscope for effervescence. This was a modification of the qualitative test suggested in ANSI/ASTM D 3290-76, "Standard Specification for Bond and Ledger Paper for Permanent Records. "
The majority of papers with a pH above 6.7, as determined by a chlorophenol red spot test, did effervesce--but inexplicably, some well-known permanent papers in a control group did not. These papers were:
The paper chemists at Mohawk and Howard, when contacted by telephone, said their papers were definitely buffered, but could not explain the absence of effervescence.
Library preservation officers and others concerned with the permanence of books and records need to be able to test the papers and books they buy to see whether they meet the specifications for permanence. Samples from larger orders of paper can be sent to a lab for standard tests; but for smaller orders and for individual books from different publishers, a spot test that works is the only thing that will tell purchasers whether they are getting long-lived paper or the other kind. The two most-used criteria for long life are pH and presence of carbonates. It may be that all modern papers with a pH over 7.0 (neutral) also contain carbonates, but this has not been established, and we cannot rely upon it. So it is important to have a spot test for calcium carbonate.
In an effort to clear up the mystery, the Editor requested lab analyses of these same control papers in 1985 and 1987 from two different paper testing labs. One lab was the Analytical Services Section at the Pulp and Paper Research Institute of Canada (PPRIC), headed by Maurice Douek. They tested three of the papers for percent of carbonate, and found wide variation between determinations on the samples, which suggests that the carbonate was not uniformly distributed in the sheet.
|
% Carbonate | |||
| Det. 1 | Det. 2 | Average | |
| 1. Mohawk Superfine | 0.84 | 1.60 | 1.2 |
| 2. Perma/Dur | 2.65 | 2.15 | 2.4 |
| 3. Howard Permalife | 1.80 | 2.87 | 2.4 |
Dr. Douek observes, "It is possible that on the samples that you tested, the amount of carbonate was too law to be detected by adding acid."
The second lab was that of a supplier of papermaking chemicals. It analyzed two samples of Paper #3 (purchased in 1983 and 1984), two of Paper #4, and one of Paper #5 (a sample received about 1980), to detect the presence and relative amounts of calcium. For comparison, they also analyzed one sample of Ecusta Waylite, which effervesces vigorously in contact with acid, and which is about 20% carbonate by weight. The method used was energy dispersive X-ray analysis (EDX), and the results were presented in the graphs reproduced below.
All of the papers contained substantial amounts of calcium. It is not known whether the Permalife analyzed by the PPRIC came from the same batch as either of the samples analyzed by the supplier, so the two analyses cannot be too closely compared. One can see, however, that the amount of calcium in the different samples below varies quite a bit, even within the same brand of paper (Permalife).
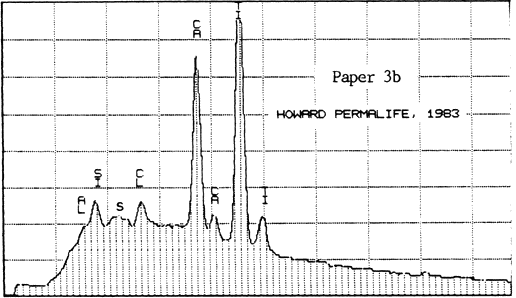
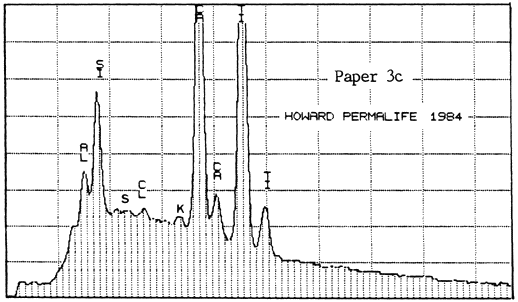
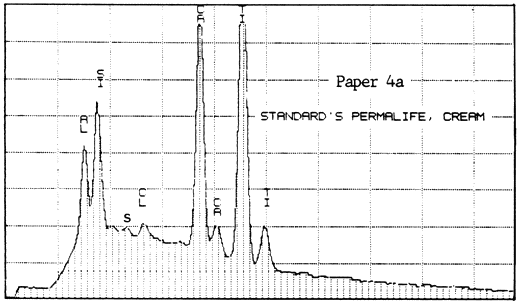
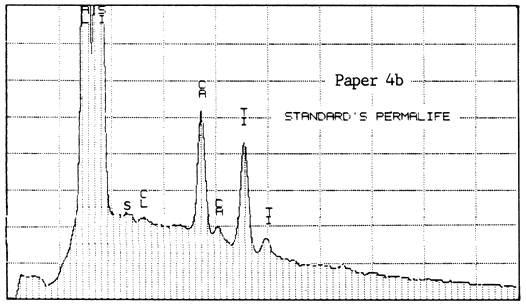
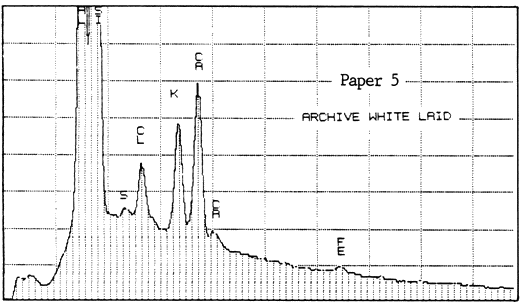
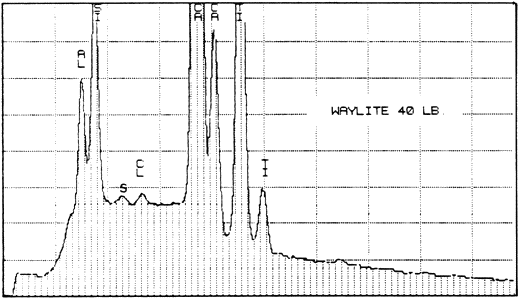
1. Because the carbonate may not be evenly distributed, the average of several large samples should be taken if an accurate figure is desired. This is true no matter what testing method is used. The tiny samples punched from the pages of library books will yield a certain proportion of false or non-representative results.
2. The effervescence-in-acid method does, however, appear to discriminate between papers containing less or more than about 3% carbonate. There is still some question whether other factors, such as size, can influence results. Therefore, this qualitative test should be used with caution. The results should be stated something like this: "____% of book papers tested positive for presence of carbonate. Because of occasional false negatives, the actual percentage may be higher than this."
3. Because pH and carbonate content are not the only factors in permanence (even though they are the only factors appearing in present-day stripped-down standards and guidelines), and because no aging studies were done on these samples, no conclusions can be drawn about the relative permanence of the papers tested.
4. Because the samples were arbitrarily selected and had been stored for years under uncontrolled conditions, they cannot be taken as representative of the papers produced today by the same companies.
To the Editor:
Re "Carbonate Content of Papers": The EDX analyses do not discriminate any Mg present, which would be buried in the low mass side of the Al peaks, but many of these carbon ate sources are diverse limestones of high silica and alumina content (as opposed to precipitated or manmade calcium carbonates) and contain (S peaks) some gypsum as well. In routine limestone analyses one also starts with 1:1 (6N) HCl [i.e., 1:1 H20 + 6N HCl] as the usual solvent, and small amounts of insoluble are assumed to be silica. But even with heating, high silica and dolomitic (high Mg) types dissolve with some difficulty. Which suggests that variability in the effervescence test may also depend on the source of the CaCO3 -
Charles Rulfs
Ann Arbor, Michigan