

A 1970 paper by A. A. Robertson in Tappi* presents a wealth of experimental data on the effects of immersing cellulose materials in various liquids. Some of that data is presented here in two graphs and one table all based on Tables I-III in the original article.
Robertson says, "When cellulose fibers or unsized papers are exposed to a liquid, a number of changes occur depending on the degree to which the liquid can penetrate the fibers and can modify the intrafiber or inter-fiber bonds. It is found, for example, that the tensile strength of paper is reduced in all liquids. In the more inactive liquids, this occurs..." without swelling. In more active liquids, swelling occurs..." the liquids he refers to as active are those closest to water on the Teas chart of solubility parameters, and those referred to as inactive are closest to heptane. In fact the nine solvents mentioned both in Table I and on the Teas chart in the Abbey Newsletter office occur in almost exactly the same order, if you draw a line on the chart from water to heptane and connect the dot for each solvent to it at right angles (Figure 1).
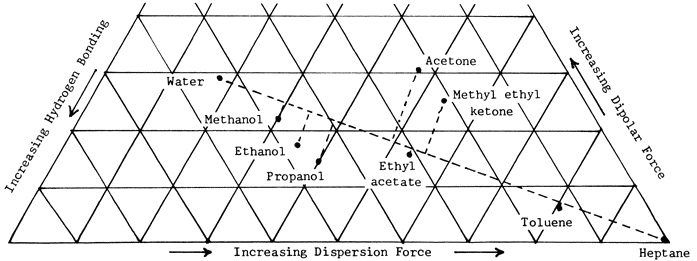
Figure 1. Distribution of selected solvents along an active-inactive (H2O-to-heptane) axis in a solubility parameter chart.
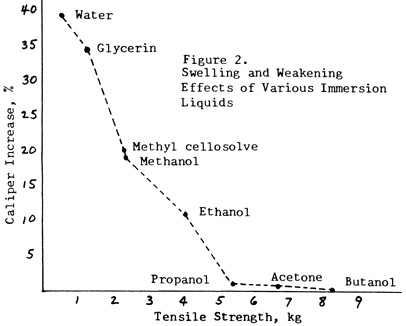
Figure 2 illustrates how the inactive liquids (propanol, acetone, butanol) will decrease the strength of paper somewhat, but will have almost no swelling effect.
Figure 3 shows how the proportion of water in such a mixture has to be reduced to about 12% in order to retain half the strength of the paper. A 1:1 mixture has almost no advantage over pure water.
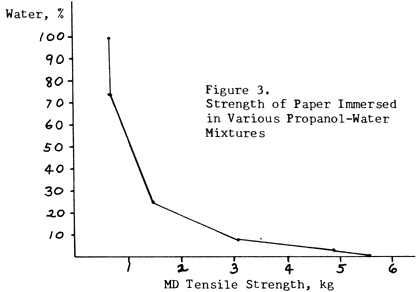
Figure 3. Strength of Paper Immersed in Various Propanol-Water Mixtures
The table in the original article from which Table 1 was taken listed 45 liquids, of which only 12 are used in book or paper conservation, according to readers' responses to last October's survey. Six more are presumably used at the Library of Congress Restoration Office, because they appear on the Teas chart used there (methyl cellosolve, butanol, ethyl acetate, benzyl alcohol and cyclohexanone).
They are listed by decreasing loss of tensile strength. This puts the most active liquids at top, and inactive liquids at bottom. It also puts the active liquids in order by the amount of swelling they induce. (Inactive liquids, those below acetone, do not induce significant amounts of swelling.)
Table I. Tensile Strength of Immersed Paper
| Tensile strength |
Immersion liquid | |
|
MD, kg |
Loss, % | |
| Water | 0.52 | 95.7 |
| Dimethyl formamide | 1.13 | 90,6 |
| Glycerin | 1.28 | 89.3 |
| Methyl cellosolve | 2.31 | 80.8 |
| Methanol | 2,35 | 80.4 |
| Ethanol | 4.05 | 66.3 |
| Propanol | 5.40 | 55.0 |
| Acetone | 6.70 | 44.2 |
| Methyl ethyl ketone | 7.75 | 35 |
| Butanol | 8.25 | 31 |
| Ethyl acetate | 8.31 | 31 |
| Benzyl alcohol | 8.40 | 30 |
| Chloroform | 9.14 | 24 |
| Cyclohexanone | 9.30 | 22 |
| Toluene | 10.20 | 15 |
| Benzene | 10.60 | 12 |
| Heptane | 11.00 | 8 |
* "Interactions of Liquids with Cellulose," Tappi 53: 13311339, 1970