 /MPa1/2 = 0.04 KB +
14.2 /MPa1/2 = 0.04 KB +
14.2 |
(5) |
Solubility Parameters: Theory and Application
John BurkeSimilar empirical methods have been used to develop other solubility scales, unrelated to the Hildebrand parameter, that quantify solvent behavior. Many of these other systems have been developed for particular applications and are appropriate for use in those applications but, although agreement between unrelated systems is somewhat loose, it is possible to correlate most of these other systems to the Hildebrand parameter. While such correlations are not always practicable, it does support the Hildebrand theory as a unifying approach, and allows the translation of solubility information into whatever system is best for the application at hand.
A particularly common cloud-point test for ranking hydrocarbon solvent strength is the Kauri-Butanol test. The kauri-butanol value (KB) of a solvent represents the maximum amount of that solvent that can be added to a stock solution of kauri resin (a fossil copal) in butyl alcohol without causing cloudiness. Since kauri resin is readily soluble in butyl alcohol but not in hydrocarbon solvents, the resin solution will tolerate only a certain amount of dilution. "Stronger" solvents such as toluene can be added in a greater amount (and thus have a higher KB value) than "weaker" solvents like hexane.
Figure 2 illustrates an almost direct relationship between KB values and Hildebrand values. This relationship is linear for solvents with KB values greater than 35 and can be expressed:
/MPa1/2 = 0.04 KB + 14.2
(5)
For aliphatic hydrocarbons with KB values less than 35, the relationship, while also linear, involves calculations that include corrections for molecular size.
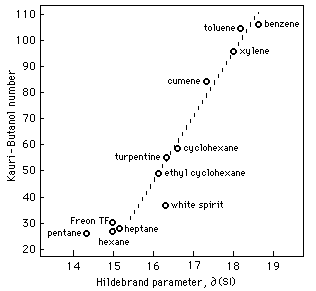
Fig. 2 Relationship Between Kauri-Butanol Number and
Hildebrand Parameter
While the Kauri-Butanol test measures the relative strength of a solvent, another cloud-point test, developed by the National Gallery of Art Research Project, is used to determine the Solubility Grade of a polymer. In this test, 10% mixtures of the polymer in n-dodecane ( an aliphatic hydrocarbon, boiling point 213°C) are diluted with varying percentages of toluene. The Solubility Grade of the polymer is the minimum percent of toluene needed to give a clear solution, thus indicating the strength of the solvent needed to dissolve the polymer. The higher the percentage of toluene in the blend, the "stronger" is the solvent strength of the blend; the Solubility Grade is therefore the mildest blend that can be used to dissolve the polymer. Table 2 gives the Solubility Grades of several polymers, along with the corresponding Hildebrand number (SI) of the toluene-dodecane solution.
| Polymer | Solubility Grade |  /Mpa1/2 /Mpa1/2 |
|---|---|---|
| Poly vinyl acetate | 89 | 18.05 |
| Poly methyl methacrylate | 87 | 18.00 |
| Acryloid® B-72 (Rohm and Haas) | 80 | 17.84 |
| Poly n-butyl methacrylate | 25 | 16.58 |
| Poly isobutyl methacrylate | 23 | 16.53 |
| Acryloid® B-67 (Rohm and Haas) | 18 | 16.41 |
| Resin AW-2 | 4 ±4 | ±16.05 |
Although The Solubility Grade gives us a conveniently broad scale for judging the solubility of polymers in mild solvents, the Hildebrand value provides a slight additional advantage: the ability to assess the solubility of the polymer in solvent blends other than toluene-dodecane. To do this, the ratio is calculated of the relative contributions of the two new solvents in terms of their distance (in Hildebrand units) from the Hildebrand value of the polymer Solubility Grade. In this way we might determine that poly isobutyl methacrylate should form clear solutions above 10% solids in a solvent of heptane containing at least 42% xylene (16.53 -15.3)÷(18.2 - 15.3). While the principle here is sound, it should be noted that the fine divisions between Hildebrand values in this instance can only give approximate results.
Other empirical solubility scales include the aniline cloud-point (aniline is very soluble in aromatic hydrocarbons, but only slightly soluble in aliphatics), the heptane number (how much heptane can be added to a solvent/resin solution), the wax number (how much of a solvent can be added to a benzene/beeswax solution), and many others. The aromatic character of a solvent is the percent of the molecule, determined by adding up the atomic weights, that is benzene-structured (benzene is the simplest hexagonal aromatic hydrocarbon). Benzene therefore has 100% aromatic character, toluene 85%, and diethyl benzene 56% aromatic character. By loose extension, the aromatic character of a mixed solvent, such as V. M. and P. naphtha or mineral spirits, is the percent of aromatic solvent in the otherwise aliphatic mixture.
These diverse solubility scales are useful because they give concise information about the relative strengths of solvents and allow us to more easily determine what solvents or solvent blends can be used to dissolve a particular material. Because most of these other systems can be more or less directly related to the Hildebrand solubility parameter, and because the Hildebrand solvent spectrum encompasses the complete range of solvents, it is the Hildebrand solubility parameter that is most frequently encountered in contemporary technical literature.
Next: Part 4 - Component Polarities