Reilly, James M. The Albumen & Salted Paper Book: The history and practice of photographic printing, 1840-1895. Light Impressions Corporation. Rochester, 1980.
| [Previous] Chapter 1 | Title Page | [Next] Chapter 3 |
| Table of Contents | Search this book |
The printer should be allowed everything of the best quality to work with, and have a comfortable room to work in.
--Edward L. Wilson, 18811
The best approach to organizing work areas is to follow the plan adopted by 19th-century portrait galleries--to have two separate working rooms, a "wet" fabrication area and a "dry" printing area in which to load printing frames and store equipment away from chemical contamination. It is preferable but not absolutely necessary that these two areas be separate rooms, but if they must share the same room, rigorous cleanliness is required. Certain requirements are common to both areas. Ideally, they should both be well lit, well ventilated, and maintained at 18-20°C and 45% relative humidity. The capacity to eliminate white light from all work areas is also necessary. Both areas need safelight illumination; for this purpose yellow "bug-lite" 60-watt incandescent bulbs work perfectly. There is no need to work in the dim light of safelights required for modern papers. The level of illumination should be bright enough to see and work comfortably. In the 19th century, windows of printing rooms were simply hung with yellow curtains or painted over with yellow paint.
The primary purpose of this area is to provide a place for the coating, drying, and processing of materials. The operations involved in coating and sensitizing should be carried on in an area that can tolerate the possibility of spills and stains. Drippings from papers hung up to dry are inevitable, and somehow silver nitrate stains always appear on floors and tables despite all precautions. A basement room is a very practical choice, providing it can be kept clean and humidity can be controlled. The level of cleanliness certainly does not have to be that of industrial paper coating facilities, but ought to be as clean as any well-kept darkroom.
Generous counter space should be available, and also adequate storage for chemicals, trays, glassware, and other equipment. Although a darkroom sink is not necessary, a sink of some kind is necessary and a darkroom type is helpful. There should also an area where freshly coated papers may be hung to dry. For safety reasons, sensitized papers should not be hung where people may pass underneath them. The floor underneath this section may be covered with plastic or newspaper to stick to wooden ones) serve well, and the lines should be strung at an angle of 5 to 7 degrees in order to concentrate runoff at one corner of the sheet. It is sometimes helpful to accelerate drying of papers with heat; if the room is small, portable electric heaters will serve the purpose. Always check to make sure that electrical wiring is adequate to handle the load created by portable hearers. Each 1250-watt heater should be placed on a separate circuit and heaters should never be left unattended.

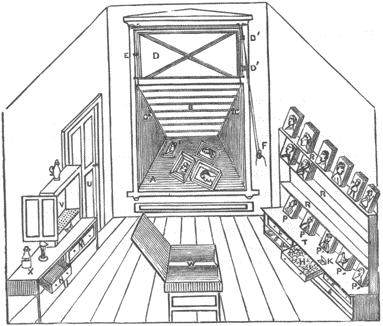
Fig. 3. A printing room, ca. 1875. Printing frames are being exposed in a window shelf. An ammonia fuming box is shown at left.
Once the printing papers have been sensitized and dried the actual operations of printing may begin, and for this purpose an area is needed that is free from the danger of splashing chemicals and airborne contamination. Counter space is required, and storage for printing frames when not in use. Daylight should be excluded from the printing room altogether, but incandescent white lights and yellow "bug-lire" safelights are necessary. It is difficult to evaluate the progress of exposure under yellow light; white lights may be turned on long enough to make the inspection. Incandescent white light is preferable to fluorescent light for inspection purposes because the chances of fogging the paper are lessened. Two drawers, one for unexposed paper and the other for exposed prints, are also helpful. If conditions of temperature and relative humidity are appropriate, then negatives may be conveniently stored in the printing room as well. The printing room may also double as a print finishing area, since this operation, too, must be kept away from chemical contamination.
Items of necessary equipment will include the following:
LABORATORY BALANCE
It is necessary to weigh out amounts of chemicals with reasonable accuracy, but a sophisticated and expensive laboratory balance is not required. A "student" grade balance with a capacity of 2000 g and an accuracy to 0.5 g will be satisfactory. A two-pan type of balance is inexpensive and convenient. Note that throughout this book the symbol g is used to mean the metric gram, a unit of weight equal to 15.432 grains.
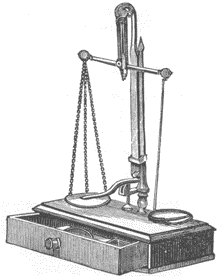
Fig. 4. A typical laboratory balance of the 1880's.
MEASURING AND MIXING CONTAINERS
For most purposes of measuring and mixing, polyethylene graduated beakers with handles are the most practical. They are unbreakable and easily cleaned. A 1000 ml and a 2500 ml model should be enough for most mixing needs. For measuring small amounts of liquids a 100 ml cylinder graduate with 1 ml graduations will be helpful. A generous supply of glass or plastic stirring rods is also necessary. A three gallon plastic or porcelain pail will come in handy if large amounts of liquids are to be mixed, and the pail may double as a dishpan for cleaning laboratory glassware. Trays and glassware should be cleaned with AlconoxTM or SparkleenTM, detergents specially made for cleaning laboratory articles.
TRAYS
Trays that have been in use for ordinary photographic procedures are usually unsatisfactory for albumen and salted paper printing because of contamination. A good approach is to buy new trays and to label them for each operation, so that there is one tray reserved for sensitizing, toning, and fixing, respectively. Porcelain trays are preferable, as long as they are not chipped in any way. Very inexpensive porcelain trays may be purchased from restaurant supply stores. While they may not be in the usual photographic formats--8 x 10, 11 x 14, etc.,--they are still serviceable, and far cheaper than trays sold for exclusively photographic use. The tray reserved for the silver solution is especially susceptible to chemical contamination, and ideally should be made of glass. If glass is not available, then a new porcelain or plastic tray will do.

Fig. 5. Glass trays, although breakable, were easily kept clean and free of contamination. From a 1901 advertisement.
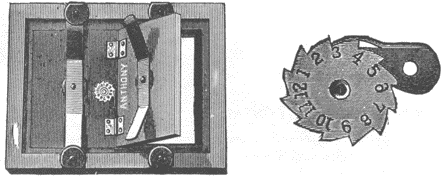
Fig. 6. "Anthony's Improved Printing Frame". A closeup view of the counter device is shown at right.
The printing frame is perhaps the single most important piece of equipment used in albumen and salted paper printing. The purpose of the printing frame is to provide a means of keeping the negative in direct contact with the paper during exposure, and at the same time allow the paper to be inspected and returned to contact in exact register with the negative. The basic design of printing frames has not changed very much since the 1840's; it consists of a wooden frame with a pane of glass in front, and a hinged back that fits inside the frame and is held in place by springs. The reason for the hinged, two-part back is so that one spring may be loosened, half of the back opened, and the progress of the exposure checked while the still-closed section holds the negative and print together to maintain registration. A felt or chamois pad is used between the paper and the back of the printing frame to evenly distribute the pressure and insure good contact over the entire print area. The use of such pads was very common in the 19th-century, but so little contact printing is done today that most people are unaware of their benefits. When printing from 8x10 or larger film negatives, pads are indispensable, especially with the poor quality printing frames available at the present time.
Printing frames should be at least one "format" larger than the negative in use--a 5x7 frame for a 4x5 negative, for example,--and they should be kept clean and dust-free. They should be of sturdy construction, with double-thickness "picture glass," not ordinary window glass, and have a good-quality laminated back for strength. Sadly, the printing frames available from photographic dealers today fall far short of those made in years past that were intended for tugged professional use. Many currently-made frames have backs of aptly-named "chipboard," which will constantly release wood splinters and spoil prints and negatives unless the utmost care is taken. It is still possible to find older printing frames made of beautifully finished hardwoods at antique stores or flea markets, or even from dealers in antique photographica, but the larger sizes of these are scarce. The older frames are a joy to use and are often less costly than their painted plywood descendants.
Many older frames have a counter device on the back. This can take the form of a notched wheel with incised numbers or may be a pointer arm that points at embossed numbers; the purpose of these counters was to keep track of the number of prints made from a given negative. With many frames and negatives in use at one time it was a handy accounting system for the quantity of prints made from each negative.
Paper
Paper suitable for albumen and salted paper printing must be of the highest standards of purity. Only papers which are "all-rag"--meaning that they are formed of pure cellulose--are acceptable. Prior to the 1920's the only way to produce a pure cellulose paper was to make it from cotton or linen rags, since the fibers of these plants are composed of cellulose in a pure form. Papers made from other plant materials, such as wood pulp, straw, or hemp, contained impurities that reacted with chemicals in sensitized coatings and speedily led to the destruction of the image. Even in the absence of a sensitized coating, impurities in such papers eventually converted to acids, which would cause yellowing and brittleness. The science of papermaking at the present time does allow wood pulp to be purified until mostly pure cellulose remains, and papers are now produced for photographic purposes which have no cotton or linen content, yet are equal to true "rag" papers in nearly every respect. Another reason why "all-rag" papers are necessary is their great strength, especially when wet; ordinary paper would simply fall to pieces if subjected to the rigors of coating, sensitizing, and processing. Even lightweight rag papers--which are preferable because they are easier to manipulate in floating steps--will not tear in processing.

Fig. 7. Cutting rags for papermaking.
Better grades of paper were made from selected rags hand cut into small squares on a special inclined knife.
In addition to the composition of the fibers, other criteria for paper selection are the color and texture of the surface. Coarse-textured, porous surfaces will tend to absorb the light-sensitive coatings, and the resultant prints may be flat and dull. If prints look better by transmitted light than by reflected light, then the solutions have sunk too deeply into the paper fibers, and either a pre-sizing step or more viscous coating is needed. If paper is tinted, the color should not be too strong and in any case should harmonize with the color of the photographic image. Paper with a smooth surface and white color is a good starting point from which to become acquainted with the color and textural qualities of various light-sensitive coatings and toning techniques.
Smooth-surfaced papers are the result of a combination of heavy sizing (sizing is the material added to the paper pulp to bind the fibers together and create a smooth surface on the sheet) and rolling between rollers to further flatten and polish the surface. If a very glossy print is desired, the paper should be as smooth as possible to begin with. Matte paper may be generated either by coating a smooth sheet with a matte coating material like starch, or by coating a porous sheet with a relatively more viscous and smooth coating.
In the 19th century, obtaining the proper paper for albumen and salted paper printing was a very difficult problem, especially for the large-scale production of albumen paper. Two firms emerged in the 1850's as producers of reliable paper for photographic use2 and dominated the world market until 1914, when the press of wartime need forced the development of new production facilities. These two mills, one in Rives, France, near Grenoble and the other in Malmedy, Belgium, supplied nearly all of the photographic rawstock consumed in the 19th century. They possessed the natural advantage of being located on mineral-free water supplies, which, together with their experience in the specialized needs of photographic paper, insured their continued success.
Many organic materials--proteins mainly but also some carbohydrates--are useful as binders or vehicles in which to disperse silver chloride and at the same time confine it to the print surface. Albumen, gelatin, starches, and whey were all in use for salted papers before 1855. Of these, albumen was by far the one most widely used in the 19th century. It is also the only one of the binders that an experienced eye may identify with reasonable certainty with just a cursory examination of a print. The following short descriptions of binder materials does not constitute an exhaustive list, and it touches only on those properties of the materials which bear on their photographic use.
Albumen for photographic purposes may be taken to mean the clear white of a hen's egg. Actually many specific proteins can be identified in egg white, but when used collectively they are referred to as albumen. Albumen has a specific gravity of 1.040, and at room temperature it dries to a brittle, transparent mass. Albumen may currently be obtained as a powder, of which a 15% solution in water will approximate native egg white, but the powdered albumen is more costly and less convenient for photographic use than albumen obtained directly from eggs.
Albumen is insoluble in alcohol, and in fact alcohol will coagulate albumen, a property that is useful to obtain multiple coatings of albumen on a single sheet. Albumen is also coagulated by temperatures above 65°C and by contact with salts of metals. The reason why albumen does not dissolve off the sheet during processing is because contact with silver nitrate in the sensitizing bath coagulates it and forms a new insoluble silver-albumen complex called silver albumenate. This substance itself is light sensitive and makes an important contribution to image formation in albumen paper.
Fig. 8. The leghorn chicken, displaying an ideal shape for . -maximum egg production.
The pH of native egg white is 7.8. Albumen is used in this fresh alkaline condition only for matte papers, and for these it is always mixed with starch or other substances. Glossy papers are prepared with partially decomposed acidic albumen, because in that condition it creates a glossier surface and more even coating, and has less tendency to yellow after sensitization. Albumen is never used in the strictly native condition; before any photographic use may be contemplated, the egg whites must be beaten to a froth and allowed to settle back to a liquid state. This beating process denatures the various proteins--all of which have different viscosities--and results in a homogeneous liquid which will form an even layer on the sheet of paper.
Like albumen, gelatin is not a specific substance but a collection of proteins. Gelatin is obtained by cooking the skins, tendons, and bones of cattle in a pH controlled vat of water. If the temperature and pH of the cooking liquor are carefully controlled, very pure forms of gelatin may be obtained. In cold water, dried gelatin swells to a viscous mass, which will melt at temperatures above 32°C. Gelatin is not precipitated by metal salts in the same way that albumen is, but additions of alum to solutions of gelatin result in gels that are harder and less permeable. Adding potassium chrome alum results in gels that are completely insoluble. Formaldehyde also exerts a hardening effect on gelatin.
Gelatin lends a characteristic reddish color to salted paper prints. The first salted papers made by Fox Talbot showed this reddish color, not because Talbot had included gelatin in his "salting" solution, but because the gelatin was already present on the paper, put there as a sizing by the paper manufacturer.
Starch occurs widely in the plant kingdom, and exists as microscopic white grains that are insoluble in alcohol, ether, and cold water. When starch is heated in water, the grains burst and a turbid paste is created. The turbidity and adhesive properties of starch pastes vary with the origin of the grains. Although many different kinds of starch may be used for photographic purposes, certain ones are preferred because the pastes they produce are pure white, very viscous, odorless, and of low turbidity. Among these the most important is arrowroot, which comes from the West Indian plant maranta arundinacea, though tapioca and sago are also useful. When starch pastes are applied to paper and dried, a layer is formed that will not swell in water and will withstand the processing solutions without damage. Starches do not react with silver salts and have no effect on the reduction of silver chloride. Most salted papers that use starch as a binder will therefore also have an "active" organic substance--usually citric acid--as part of the formula.
Resins are noncrystallizable, amorphous substances which are obtained from plants, notably the sap of softwoods such as pine and fir. Resins are distinguished from gums by their insolubility in water. They are soluble in alcohol, ether, etc., and such solutions are known as lacquers. When mixed with alkalies such as ammonia or sodium hydroxide, they form foamy solutions known as resin soaps; if acids are added to resin soaps, the resin is precipitated in an insoluble form. Resin soaps are also precipitated by contact with silver nitrate solutions, forming an insoluble white mass that is moderately light-sensitive.3 The presence of resins has no effect on the reduction of silver chloride.
| [Previous] Chapter 1 | Title Page | [Next] Chapter 3 |
| Table of Contents | Search this book |