van Monckhoven, Désiré van. A Popular Treatise on Photography. Translated By W.H. Thornthwaite. London, 1863.
| [Previous] Chapter 1 | [Next] Chapter 3 | Title Page Preface Introduction |
| Table of Contents | Search this book | Albumen Home |
A MIXTURE of alcohol, sulphuric ether, and gun-cotton forms a liquid called plain collodion, to which is added, to render it suitable for photographic purposes, an iodide or bromide; it is then termed iodised or sensitised collodion.
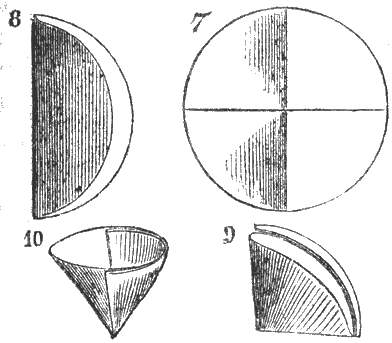
Spirits of wine, or alcohol, is a liquid well known, and can generally be procured sufficiently pure for photographic purposes., it boils at about 172° Fahr., and burns with a bluish flame without leaving a residue. One hundred ounces by weight of alcohol measure about 125 fluid ounces, and 100 fluid ounces weigh about 80 ounces. It should be perfectly clear, transparent, and absolutely free from any floating impurities; should it be otherwise, it must be carefully filtered. The operation of filtering, applicable to other liquids as well as alcohol, is thus performed. A circular sheet of filtering paper is first folded in two, as represented by Figs. 7 and 8; then a new fold is made in the middle, Fig. 9; the filter is then opened out, as shown at Fig. 10, and placed in a funnel, b furnished with its support, Fig. 11. The alcohol or other fluid to be filtered, is poured carefully into the filtering paper, through which it will pass perfectly clear. Should the first portion that runs through not be quite bright, it must be returned to the filter. When a very rapid Fig. 12. Plaited filtration is desired the filter paper may be folded into a number of plaits, as shown at Fig. 12, which affords a larger surface of paper for the liquid to pass through.
Figs. 7, 8, 9, 10. Method of folding Filtering
Fig. 11. Apparatus for Filtering Papers.
Fig. 12. Plaited Filter.
Figs. 13, 14, Hydrometer and Glass
The strength of alcohol is conveniently ascertained by a specific gravity hydrometer. This instrument is formed of glass, Fig. 14. It consists of a glass bulb with a glass stem attached at the top, and a smaller bulb filled with mercury, to serve as a counterpoise at the bottom. In the stem is placed a graduated scale of specific gravities, and the whole is so arranged that when placed in pure distilled water, the instrument floats, and the surface of the water is coincident with 0 or 1000 on the scale. When placed in alcohol or any fluid lighter than water, the hydrometer sinks according to its strength or specific gravity. A test-glass or cylinder, Fig. 13, is used to hold a sample of the alcohol or other liquid to be tested, and care must be taken when the hydrometer is placed in it that it floats perfectly free in the fluid, and that no air bubbles attach them selves to its surface. The specific gravity is. then immediately indicated by noting the degree cut by the surface of the fluid. alcohol, suitable for photographic purposes, should not have a greater specific gravity than .819. Its best strength is about .803 to .810.
It is comparatively easy to procure alcohol of sufficient purity for photographic use, to what it is to obtain pure sulphuric ether. When manufactured on a large scale for ordinary pharmaceutical purposes, there is generally so
little care taken, that the ether becomes contaminated with sulphovinic acid aldehyde, or, worse than all, a peculiar principle resembling ozone, which is capable of decomposing iodides and liberating free iodine, sherries, highly detrimental to its photographic action, The formation of this substance is touch accelerated by the joint nation of air and light; the knowledge of this circumstance is, therefore, of soma importance to photographers, as it indicates a very necessary precaution to be taken to keep ether, mod liquids containing it, particularly collodion, it yell filled and closed bottles.
Sulphuric ether is a colourless liquid, and very volatile; whom poured into water it boats about like oil, and a very smell quantity is dissolved. It is much lighter than water, 10, ounce. by weight of ether being equal in volume to 106 ounces by weight of water. Ether is tested to, to its strength by the specific gravity hydrometer, in the same manner as described for alcohol on the opposite page. It should have a specific gravity of .750 to .720 to be of any use in photography.
In consequence of the highly volatile nature of ether and its vapour being very explosive when mixed with ,atmospheric air, it is necessary, to prevent accidents, to avoid pouring ether from one vessel to another in a close room, or in proximity to a fire, or flame. As the vapour of ether is much heavier than the air, it naturally tends to fall, and therefore it is n proper precaution to take, when employing ether or fluids containing it--as collodion, for example--by artificial light, to have the source of light situated at some distant, above the vessel from which the ether or collodion is poured.
Ether, if not sufficiently pure for photographic purposes. can generally be made available by the following method of rectification:--
Into a tall bottle, Fig. 15, is to be poured the ether to be purified, together with one-fourth of its volume of water, and the opening closed with a cork; the whole is them strongly agitated, and left to settle for some few minutes. Two layers of liquid will be perceived the under layer being water slightly etherised, and the upper ether. The cork is now removed, and the shorter end of an ordinary glass syphon, having a small bore, and previously filled with water, is introduced through the neck of the bottle, and quite to the bottom of the liquid. The smallness of the bore, and keeping the finger over the longer end of the tube, will enable the above to be done with facility, without the water from the syphon running out. The finger being removed, the syphon begins to act, and the etherised water from the bottom of the bottle is quickly drawn off. When the under layer has nearly disappeared, the orifice of the tube is again stopped with the finger, and the syphon removed.
Fig. 16., Fig. 15.
A fresh quantity of water is now poured into the bottle containing the ether, which is again agitated and drawn off by the syphon as before explained.
This operation is called "washing," and the ether after this process is called "washed ether."
Fig. 17. Bottle with underlayer of liquid has passed over, the Syphon.
If bent glass tubes can be conveniently made or obtained, the following arrangement may be found more convenient than the ordinary syphon, it is shown at Fig. 17: A, the bottle where the ether and water is shaken together; it is furnished with a good cork pierced with two holes, in one of which is fitted a narrow tube (a) about 3/8ths of an inch internal diameter, and in the other, a curved syphon tube (b), of which the shorter end inside the bottle reaches to the bottom. If the cork be properly fitted, it is only necessary to blow slightly through the tube (a) to cause the liquid to rise in the tube (b) and flow over. When nearly the whole of the syphon is stopped with the finger, the cork removed, and the fresh quantity of water added, and the operation gone through a second time.
The ether having been well washed, now requires to be dried and distilled; this is done by pouring the ether remaining in the washing bottle into a distilling weasel containing some few pieces of quicklime.
Fig. 18. Apparatus for distilling Ether.
A convenient arrangement of apparatus for the distillation of small quantities of ether, is shown at Fig. 18; where larger quantities are operated on, the;lass retort should be replaced by a vessel of zinc or tin plate. A is a small furnace for charcoal, B a vessel of copper or iron of some convenient form, to hold a small quantity of water, C a glass retort or other vessel, the opening of which is attached, by means of a cork, to a small leaden tube abort the thickness of the little finger, and 1½ yards long; a portion of this tube is surrounded by another about 2 inches in diameter, and ¾ths of a yard long; the top and bottom of this tube is closed perfectly water-tight round the smaller tube, it has also an overflow tube (a) at the top part, and a funnel and tube (b) at the bottom, through which a stream of cold water can be passed from any convenient vessel, as F, and discharged into the receptacle H. The end of the small leaden tube is bent so as to dip into a perfectly clean bottle (G); in every other respect the figure will convey a correct idea of the construction of the apparatus.
When about to be used, each separate part of the apparatus should be perfectly cleaned and washed out with water, and arranged as described and shown in the cut. The glass retort (C) or other vessel, is filled for about one-fourth its volume, with small pieces of quick lime, and the washed ether poured on to it until two thirds of the bulk of the retort is filled; the end of the leaden tube is then attached to the neck of the retort, and. the refrigerator E D arranged in an inclined position, and firmly fixed by its support (d) so that the bent end of the tube dips into the mouth of the bottle G, which is to receive the distilled ether. The whole being thus arranged, a small quantity of water is poured into the vessel B, so that the lower portion of the retort C is immersed in it, forming what is called a water bath some lighted charcoal is now placed in the furnace fl. and the water in the vessel B becoming heated, communicates its beat to the ether in the retort, which begins to evaporate, and in a short time drops of ether appear at the bottom of the leaden tube, and the distillation begins.**
The water in the vessel B gets more and more heated as the bulk of ether in the retort diminishes, until no more drops are perceived to fall from the end of the tube; the fire is now removed, the apparatus separated, and the retort (C) or other vessel at once cleaned out, for should this be delayed, it becomes very difficult of performance.
The heat of the fire must be kept as much as possible from the bottle (G) containing the distilled ether, and a current of very cold water passed through the refrigerator E D, otherwise the ether vapour is not condensed. Sulphuric ether, rectified in the manner described, although not absolutely and chemically pure, is nevertheless well adapted for photographic purposes.
3. Gun-cotton.
Gun-cotton, also termed "pyroxyline," is nothing more than ordinary cotton combined with peroxide of nitrogen. It can be prepared by plunging cotton wool for a few minutes into concentrated nitric acid, then washed in water and dried; but in order to obtain a good pyroxyline for photographic purposes, a particular process must be followed, and a rigorous attention paid to each separate detail. Gun-cotton in appearance much resembles ordinary cotton, but it is heavier, and its fibres break more easily; it possesses also a slightly yellow tint, which resembles that of raw cotton as imported into Europe from the colonies. It is insoluble in water, alcohol, pure ether, sulphuret of carbon, or chloroform, but it dissolves in acetate of ethyle and methyle, methylic alcohol, acetone, and also in alcoholised ether.
Pyroxyline burns with violence when brought in contact with any flame; so much so as in many instances to answer the purpose of common gunpowder.
The solution of gun-cotton in alcoholised ether is called collodion, and is employed in surgery and photography; but for this latter purpose it requires to be specially manufactured.
The following is the method of preparing gun-cotton for photography, although we strongly recommend its being purchased ready made, as photography being now so extensively employed, gun-cotton is prepared on a large scale, and at a low price.
In a porcelain mortar is placed 2 ozs. of saltpetre in fine powder, and over it is poured 3 ozs. by weight of sulphuric acid of commerce. With the pestle, or a large glass tube, the materials are well mixed, so as to obtain a homogeneous paste. In this is immersed, in successive portions, ¼ oz. of carded cotton, free from any mechanical impurities. The cotton is pressed down with the pestle until thoroughly wetted and imbedded in the liquid paste. The mortar is then covered with a plate, to prevent the nitrous vapours which are given off from vitiating the air of the laboratory. It is also advisable to perform this operation, if possible, in the open air.
The cotton is left in this mixture for ten minutes; the mortar is then placed in an inclined position, and water poured into it, at the same time pressing the cotton with the pestle so as rapidly to remove the excess of acid. After washing for a half minute in this manner, it is taken up with the hands and thrown into a wooden tub filled with water, and well kneaded; or else held under a watercock, and constantly worked about, and from time to time pressed strongly between the hands. This washing should be thoroughly done, until a portion of the cotton, when put, in contact with blue litmus paper.1 does not produce a red stain. It is then strongly pressed, and left to dry in the air or in the sun, having previously spread it oat thinly, so as to present a large surface to the air. When the cotton is dry it is preserved in glass bottles, well stopped. Gun-cotton, thus prepared, very often gives traces of sulphate of potass; but as this substance is absolutely insoluble ether and alcohol, it is of no importance.
Large quantities of gun-cotton should not be bought or ,prepared at one time, as it appears to be liable to decompose by keeping.
Gun-cotton, or pyroxyline, can be prepared according to the formula given above from paper, linen, or hemp; but these preparations have not been sufficiently studied for us to recommend their employment in photography.
At the end of this volume3 Some details are given relative to the manufacture of gun-cotton. on a large scale by a mixture of nitric and sulphuric; acids. In ,general, the gun-cotton so prepared is less soluble than that which has just been described; it, however, yields an excellent collodion, especially adapted for coating large plates, from its being very adherent.
Gun-cotton was discovered by M. Schonbein, a German chemist ill 1846. The photographic process which employs collodion as its basis was first described by Mr. Archer, in England, in 1861. M. Schonbein prepared bun-cotton by steeping cotton in monohydrated nitric acid. Afterwards M. Meynier discovered the advantage of using a mixture of concentrated nitric and sulphuric., acids, and the method of preparation with saltpetre and sulphuric acid is clue to M. Marc Antoine Gaudin, calculator in the Bureau des Longitudes of France.
In connection with the method of preparing collodion, presently to be described, will be indicated some other important points as further guides to the selection or manufacture of a good gun-cotton.
A great number of iodides, bromides, and their compounds have been at various times proposed for sensitising collodion, but the formula most to be recommended is a mixture of iodide and bromide of cadmium. In Note 4 will be found some remarks on the employment of the iodides and bromides of potassium and ammonium.
Cadmium is now easily procured, almost, in a pure, state, and at a comparatively cheap price. This metal is generally found in commerce in small cylindrical ingots, about four inches in diameter, and one-fourth in diameter. Its purity can be known by its making a ringing crackling noise when bent, like tin. If it bends with difficulty, and produces no sound when bent, it contains some other metals, usually copper and zinc.
Iodine is a crystalline substance, having the aspect of black-lead, or plumbago, volatile at a slight increase of temperature, giving off purple vapours, highly corrosive, and irritating to the eyes; it should always be preserved in glass -stoppered bottles. It is obtained from the ashes of burnt sea-weeds.
Bromine.--This substance is obtained froth sea-water, after all the common salt has been removed by boiling. It is a very dense, dark, red liquid; its vapour is highly injurious and corrosive, and, froth its great volatility, is always kept under a stratum of water or sulphuric acid, and in glass-stoppered bottles. Both iodine and bromine are easily procured from any chemist.
Iodide of cadmium is thus prepared:-In a glass flash-, containing a quart of water, at first put in 8 ozs. of iodine, and immediately after 4 ozs. of cadmium in small pieces. The flask is placed on a stove, moderately heated, in such a manner that the water in the flask shall be kept only warm, not boiling. At the end of a few hours, especially if shaken from time to time, the liquid, from red, which it was at first, will become entirely colourless. Leave it to cool, and then filter. The cadmium that remains may be used for another operation.
The solution of iodide of cadmium thus obtained, is evaporated in a porcelain capsule. After a certain time crystals will appear in the liquid. It is then placed on a very hot stove, where all the water is driven off, and a dry mass obtained. The resulting substance is detached from the capsule with a knife, then reduced to a fine powder in a mortar, and finally preserved in a stoppered bottle.
The iodide of cadmium thus prepared is of a yellow tint, very soluble in water and alcohol, but less soluble in ether.
Bromide of cadmium is made by pouring 6 ozs. of bromine into 1 quart of water, contained in a stoppered flask; 4 ozs. of cadmium, in small pieces, are now added, and the flask closed. This mixture is left for some days, and very carefully shaken from time to time; the liquid gradually becomes discoloured, from the absorption of the bromine; when this takes place it is filtered and evaporated to dryness, as described for iodide of cadmium.
Bromide of cadmium is of a white colour, and less soluble in water and alcohol than the iodide. These substances, when prepared for sale on a large scale, are obtained beautifully crystallised, which may be taken as an evidence of their purity.