

van Monckhoven, Désiré van. A Popular Treatise on Photography. Translated By W.H. Thornthwaite. London, 1863.
| [Previous] Chapter 15 | Title Page Preface Introduction | |
| Table of Contents | Search this book | Albumen Home |
1. Blue litmus paper is turned red by acids; red litmus paper is turned blue by alkalies. These two papers can be bought ready prepared, or they can be made in the following way:--Half a pound of litmus, in small cakes is boiled in an iron vessel with one quart of wafer for some minutes, and then poured through a line piece of linen, to separate the undissolved portion. This solution is spread over paper by means of a camel's hair brush, and the blue paper thus obtained hung over a cord to dry. To make; the red paper, a small quantity of vinegar is added to the foregoing blue liquid until it becomes of a reddish colour. It is best to cover the paper on both sides, and to cut it into small hands, which should be kept in closed bottles, so as to prevent the action of acid or alkaline vapours.
2. This remark is correct only as far as regards the practice of continental photographers,-in England glass and porcelain baths are always employed.
3. View lenses are sometimes mounted, with the diaphragms or stops fixed in the large tube bolding the lens, which either slides in another tube or is actuated by rackwork, to facilitate the obtaining a correct focus.
4. In the most recently improved form of compound lens, the sliding body is pierced so as to allow a series of diaphragms, or stops, to be introduced between the two lenses, as shown in the illustration Fig. 54. This arrangement is more correct in principle, and produces better results, than the simple external stop.
5. Those rays of light which produce chemical action, and are found at the blue and violet end of the spectrum.]] are cut off, and the exposure necessary for a good portrait is greatly augmented.
6. M. de la Blanchère gives the following formula:
| Peroxide of Iron, or Rouge; Chromate of Lead, or Ivory Black | 10 parts. |
| Gum Arabic, Saturated Solution | 2 parts. |
| White Honey | 2 parts. |
| Sugar Candy | 1 part. |
The difference of the reverse appearance of an ordinary negative on glass or paper, and the direct one exhibited by a metal plate, is simply apparent and not real and arises solely from the mirror-like appearance in those parts of the silver plate not acted on by light. And this explains the reason why it is necessary to give to these (daguerreotype) pictures a particular position in relation to the angle at which the light strikes them. At other angles, when, for example, the light is directly reflected from the surface of the metal, the image appears as a negative.
The object aimed at in the distillation of a liquid is the separation of any solid substance which it holds in solution or any liquid of a different constitution with which it may be mixed. In the instance under consideration, it is necessary to separate the ether, not only from the chloride of calcium upon which it has been dried, but also from the alcohol and water which it contains.
The boiling point of ether being about 96° Fahr., and of alcohol 174° Fahr., and water 212° Fahr., it follows, that if a mixture of the three be submitted to the action of heat, the ether will be almost completely volatilised before any sensible evaporation of the alcohol or water has taken place. A tube thermometer, having its stem passed through the cork, and its bulb so arranged as not to come into contact with the liquid, will indicate a temperature of about 96°, rising higher in proportion as more alcohol and water become evaporated. To secure pure ether, the temperature should not rise higher than 10°.
The first portions which come over should not be used, as they gene. rally serve only to clean the condensing tube, and, consequently, contain impurities. From two pints of ether, therefore, the first ounce and a half which comes over should be rejected.
The cotton should be chosen free from defects and any contaminating organic matter. A mixture is then made of
Sulphuric Acid (Sp. Gr. 1.8) 88 fluid ounces.
Nitric Acid (Sp. Gr. 1.4) 19 do. do.
This is stirred with a glass rod, and if examined by a thermometer will be found to indicate a temperature of 176° Fahr.; the operator should therefore wait until it cools down to 140° Fahr. before plunging in the cotton.
The quantity of cotton to use is about 1,050 grains, which is added to the acids, about one-fourth or one-fifth part at a time, squeezing it with the glass rods in order to force out the air imprisoned between the fibres. When all the cotton is immersed, the containing vessel is covered with a plate to keep in the nitrous vapours, and at the end of ten minutes the cotton is withdrawn, and copiously drenched with water, as before described. The pyroxyline thus obtained is less soluble in ether and alcohol than that obtained by the ordinary method; but it is especially useful when great tenacity of film is required.
Besides iodide and bromide of cadmium, a great number of other iodides have been used, among which may be mentioned those of potassium, sodium, ammonium, zinc, &c.
Many photographers confine themselves to the use of the iodides of potassium and ammonium, but lately iodide of cadmium has come generally into use.
More recently has been proposed, especially for copying pictures, a collodion, containing iodide, bromide, and chloride of ethylamine. The following is the formula which has given the best results:
| Alcohol | 1¼ ounce. |
| Ether | 2½ ounce. |
| Pyroxyline | 15 grains. |
| Iodide of Ethylamine | 1.2 grains. |
| Bromide ditto | 0.4 grains. |
| Chloride ditto | 0.2 grains. |
Although iodide of ethylamine is not found in commerce, it is very easy to prepare. It is an organic iodide, containing nitrogen, and the elements of alcohol, but is nevertheless more stable than iodine of ammonium, and yields pictures of remarkable delicacy.
Nitrate of silver is reduced by contact with all organic substances, and as finely-divided metallic silver is black, it follows that this substance blackens everything it touches, as every photographer knows. Many methods have been proposed for the removal of these spots. The following is the best.
The hands become inevitably stained while conducting the manipulations involved in the sensitising and development of the plates, but it is not until some hours have elapsed that the stains, at first scarcely visible, deepen to any great extent; and it is only when a great number of proofs have been taken that the method about to be proposed is required or advisable.
It consists simply in well washing the hands in a saturated solution of hyposulphite of soda, kept expressly for that purpose. Two or three minutes' contact, are found sufficient to remove every trace of the stain. Instead of hyposulphite of soda, iodide of potassium may be used in the same way. After the hyposulphite, it is advisable to wash the hands with soap, and with powdered and sifted pumice stone.
If the stains are very old, it is better to allow them to wear away by time; however, as they sometimes are obliged to be removed, in that case, a mixture of cyanide of potassium and iodine applied to the fingers by a brush, or otherwise, will, if aided by the use of a lump of pumice stone, rapidly restore the hands to their normal condition, after which they should be well rinsed with plenty of water.
In the use of this latter re-agent for the purpose indicated, it is important to remember that cyanide of potassium is an energetic poison which acts not only internally, bat externally, by absorption; so that it should never be used when there is the slightest wound or scratch upon the hands.
If this advice be neglected, serious results may follow. It is also advisable that this mixture should only be prepared at the time of using it and that every vessel which has contained it should be carefully cleaned.
Into a graduated glass is placed 1,200 grains of nitrate of silver, upon which is poured 7 ounces of distilled water. When the nitrate is all dissolved, which can be hastened by well stirring with a glass rod, there is to be added 3 drams of an alcoholic solution of iodide of cadmium, containing 10 grains to the ounce; a yellow precipitate is immediately produced. The whole is well agitated together, and left to itself for about one quarter of an hour; more distilled water is then added to make up the quantity of 35 ounces; it is then filtered, and is ready for use.
This substance was discovered by Scheele, who supposed it to be sublimed gallic acid. It contains the elements of gallic acid, minus those of carbonic acid.
Dry and pure pyrogallic acid has the form of lamellar needles, or elongated plates which are soluble in 2. parts of water at ordinary temperature, and a little less soluble in ether and alcohol. It has a very bitter taste, and when quite pure, does not redden tincture of litmus. A solution of the pure acid will keep, so to speak, indefinitely, especially if, as is the case when it is prepared for photographic use, an acid be added.
It is of the highest importance to keep pyrogallic acid in stoppered bottles in the dark; since unless this be done it gradually turns brown, through combination with the oxygen of the air, and its properties injured.
Pyrogallic acid is prepared by boiling bruised nut-galls, with seven or eight times their weight of water, for three or four hours, replacing the water as fast as it evaporates. The whole is then thrown on a strainer, and the dirty cake of nut-galls being submitted to powerful pressure, in order to remove all the liquid.
The mixed liquors are then evaporated, first, by rapid ebullition, and afterwards more gently, until they acquire the consistence of an extract, which extract is rendered perfectly dessicated by careful drying in a stove.
This product is then heated for ten or twelve hours in a flatiron vessel, over the top of which is stretched a piece of perforated paper, the whole being covered by a conical paper cap. The vessel is placed on a sand-bath, and the temperature, which should not rise higher than 420° Fahr., is indicated by one, or, still better, two thermometers. And this is the most delicate part of the operation. If the heat be insufficient, no result is obtained, and if it be too much heated, another product is obtained which contains no pyrogallic acid.
Operating in this way, 100 parts of dry extract yield 5 parts of pure pyrogallic acid, and at a more advanced stage of the sublimation, 5 parts of impure acid, which may be purified by resublimation.
The reader will readily understand that it is by no means easy in so limited a work as the present to give a clear idea of photographic optics; the observations to be made will therefore be confined to demonstrations of the fundamental principles only of the most advanced of the sciences.
The ultimate constitution or essence of light is entirely unknown. Newton entertained the idea that luminous bodies threw out in all directions exceedingly minute corpuscles, which, on coming into contact with the optic nerve, produced a certain effect, which has been distinguished by the term " luminous effect." It has, however, been since proved that the hypothesis of Newton does not accord with facts, and the view most generally entertained is to the effect, that there exists in space permeating our atmosphere and all existing bodies, a luminous ether of extreme tenuity, to which luminous bodies have the property of communicating a vibration similar to that which takes place when a spring is suddenly struck; and it is this vibration, communicated with an almost inconceivable rapidity to the optic nerve, which produces the effect which is called light.
It is not certainly known whether this last hypothesis is a correct one, but it is certain that it adapts itself most completely to all the known facts of optics, and that it has materially aided in the discovery of some of them.
It is well known that light travels in straight lines at a rate of 240,000 miles in a second of time.
Reflection of light is that effect which takes place when light falls on a plane mirror.
The light which falls on a mirror from a luminous point is called the incident ray, and that which reaches the eye from the mirror is called the reflected ray; and if a perpendicular be elevated from the point where these two rays meet, it will be found to make two equal angles, or, in other words, the angle of incidence and the angle of reflection are equal.
Reflection takes place with equal regularity from the surface of curved mirrors, but the image is modified according to the character of the curve, which sometimes enlarges, sometimes diminishes, sometimes reverses the image, and sometimes gives an erect image.
Refraction is that phenomenon which is shown when a stick is plunged into water, by its appearing as though it was broken. It is also a well-known fact, that the bottoms of rivers appear to be a great deal nearer than they really are, and that in consequence great mistakes are sometimes made in judging of their depth; this phenomenon also belongs to refraction. In one word, refraction of light takes place whenever a ray of light deviates from its original course by passing through a transparent body of greater or less density.
In the first place will be considered the most simple case (Fig. 98), in which a ray of light R is incident on a plate of glass A, whose two sides are parallel. At the point where the ray R comes into contact with the glass, imagine a perpendicular line to be drawn, and then observe what happens to the luminous ray. (The perpendicular here mentioned is distinguished by the term normal Experience has shown that the angle R N, formed by the luminous ray and the normal ray, is greater than the angle formed by the same ray in the interior of the glass with the same normal ray prolonged; but this angle is constant for the same quality or character of glass. On leaving the glass the ray assumes a direction parallel to the direction of the primitive ray.
It follows then from what has been stated, that a ray of light falling on the surface of a piece of glass is refracted both on entering and leaving the glass.

| 
| 
|
| Fig. 98. | Fig. 99. | Fig. 100. |
Figs. 99 and 100 illustrate the same fact, and also show the reflection of a ray from the large face of a triangular prism.
Fig. 101.
The study of refraction becomes more difficult when the two surfaces of glass instead of being parallel, are placed at an angle in relation to each other (Fig. 101). Suppose two of these faces A C, A B, and a luminous ray Ro falling on one of the surfaces. If it were not a prism, the ray would follow the direction R M; but as the ray Ro is bent, as in the preceding case it approaches towards the normal in the direction o o', the angle outside the prism being greater than the angle inside. But the reverse happens on the second face A B, at the point o' and the ray leaves in the direction o' R' in such a manner that the ray, first impelled in the direction R M, is finally sent in the direction o' R', from which it follows that a ray of light is altered in its direction by its passage through a piece of glass, the two sides of which are not parallel.
Fig. 102.
Fig. 103.
Fig. 104.
Glass lenses are convex discs, ground and polished, having an exterior figure of a spheroidal character. Their principal effect is, that when they are exposed to the sun, the parallel rays of that luminary r r (Fig. 102), unite at a certain point, called the focus, all the rays converging towards this point. Inversely, a luminous point being placed in the focus f of a lens A, emits rays which, on leaving the lens, are parallel r r r r. It therefore follows, that a convex lens is in effect an assemblage of prisms, the inclination of the two faces of the glass from the centre to the circumference being such that the parallel rays undergo an analogous deviation, which causes them to meet at the focus. Fig. 104 is intended to illustrate this proposition.
Fig. 105
In concave lenses the effect is entirely different. If solar rays r r impinge on a concave lens A (Fig. 105), these rays, instead of uniting, disperse themselves in the direction r' r'. It is, however, usual to regard the point f as the focus, resulting from the ideal prolonging of the rays r' r' and focal length the distance A f.
If refraction consisted only in a simple deviation of the rays of light, it would be a comparatively simple matter, but unfortunately, it is not so; the ray is not only bent, but decomposed into its primary colours.
This statement may be easily verified by examining a white object on a dark ground through one of the ordinary prisms used for ornamenting chandeliers. The white object will appear fringed with all the colours of the rainbow.
| 
|
| Fig. 106. | Fig. 107. |
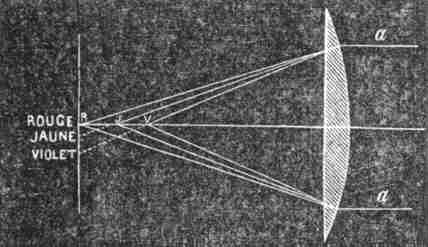
The same phenomenon occurs when a ray of light R (Fig. 101) is allowed to fall on the surface of a prism. The refracted ray o' R' consists not of one white ray, but of seven different colours. Convex lenses produce the same effect. Thus, the solar rays a a falling on a convex lens (Fig. 106) do not reunite in one single point, but produce at It a white image, bordered above by red, and below by violet.
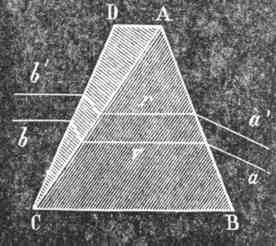
It is possible, however, to unite two prisms (Fig. 109) in such a wag that the rays b' b falling thereon, shall leave it at a' a void of colour; such a combination of glasses is called an achromatic prism. These prisms are composed of two kinds of glass-crystal, or flint, and crown, or ordinary glass.

| 
| 
| 
|
| Fig. 108. | Fig. 109. | Fig. 110. | Fig. 111. |
There are not only achromatic prisms, but lenses, which yield images free from colour. The curve which a particular kind of glass is to receive in order that the compound lens shall be most effectually achromatised, forms the subject of a mathematical calculation. The curve is then imparted by grinding the glass by hand in a suitable tool.
There are many different kinds of lenses. Fig. 108 shows three forms of non-achromatic convex lenses and Fig. 110 these same lenses achromatised. Figs. 109 and 111 concave lenses non-achromatic and achromatised. In order to explain the application of lenses in photography, it becomes necessary, in the first place, to describe a very curious phenomenon.
In looking at a well-lighted landscape, it is obvious that every point of that landscape sends to us a ray of light, since, unless it were so, it could not be seen at all.
Fig. 112.
If a hole be bored in the shutter of a perfectly dark room (Fig. 112), and a sheet of white paper be placed a short distance from the aperture, it will be seen that, as every point of the view emits luminous rays, a certain number of these rays will pass through the opening into the dark chamber, and being directed by the sheet of white paper, will produce thereon an inverted image of the landscape.
If a convex lens be placed in the hole in the shutter, and the sheet of white paper brought up to the focus of this lens, the image will be increased so much in sharpness that it will seem quite easy to trace the outline with a pencil, or, indeed to make a finished drawing. Now, if the sheet of white paper be replaced by another sheet of paper photographically prepared with some substances acted on by light-as the compound of silver, for example-the image of external objects will, in a longer or shorter time, be depicted thereon.
In Fig. 106 has been shown the remarkable fact, that the two white rays a a become decomposed into rays of various colours; but what is still more curious is, that if the rays a a were red, they would come to a focus at R; if yellow, nearer the lens at J and if violet still nearer at V. In one word, a lens acts equally only for light of one colour, and unequally on different colours. Lenses of the same form but of different glass, will also act differently on the same light; and it is precisely on this account that it becomes necessary to make a combination of glasses of such forms that all the colours shall be equally refracted and reunited in one point.
For this purpose lenses are made and placed very near to each other, in some instances even united by Canada balsam, one lens, so to speak, for each colour; generally, however, the combination is confined to two lenses uniting only the two principal colours.
In the construction of photographic lenses, the relation between the material, i.e.--the quality and kind of glass-and the form or curve imparted to it is calculated in such away as to unite into one focus the yellow and the violet rays.
Fig. 113.
Sometimes double and sometimes single objectives are employed. The double objective is composed of four lenses mounted in brass, Fig. 53 represents such an one, and Fig. 113 the arrangement of the lenses, The point of the arrow is directed towards the object. to be taken. This system of four lenses is arranged in such a manner as to give a great deal of light to the image, to the sacrifice to some extent of sharpness. A single achromatic lens may, however, be employed which, while it gives less light, wonderfully increases the delicacy of the details.
It is not intended to describe all the kinds of spots which are produced on the collodion film, but only those which occur most frequently.
Spots are sometimes produced under the collodion film, and sometimes upon the film. The first, always visible before exposure, arise generally from imperfectly cleaning the plate. In fact, the dust which remains on the plate are centres of reduction for the iodide of silver constituting the film, and thus form round spots.
Another source of spots arises from the presence of fatty particles in the leather used for cleaning, and these produce stains such as shown at page 63 (fig. 65).
In reference to spots produced on the film, they arise very often from light solid bodies floating in the collodion. It is, therefore, of the highest importance always to use a collodion which has stood some time.
It happens sometimes that the little crystals of iodo-nitrate which float in the nitrate bath deposit themselves on the film, and on this account some photographers pour their silver solution from a bottle, into which it has been filtered, after sensitising a considerable number of plates.
At other times the bottom of the plate is riddled with holes. These are caused by the concentration of the nitrate on account of the plate having been kept too long between the sensitising and the development.
Sometimes when the nitrate bath has not been filtered for a long time a pellicle of reduced silver is formed which attaches itself very firmly to the film. Veins also occur, which are aptly represented by fig. 66, page 63.--(M. de la Blanchere.)
A similar class of stains sometimes occur, especially on positives if the bath contains an excess of alcohol and they become visible on withdrawing the plate from the sulphate of iron solution.
The stains shown in fig. 67, page 63, arise when too thick a collodion is used.
Pyrogallic acid often produces spots. If the developer prepared therewith contains too little acetic acid, foggy pictures are the result. If it contains too much, the development proceeds very slowly. But spots occur less frequently in the latter case than the former.
Should too small a quantity of pyrogallic be poured on the plate, stains develop themselves at the corner, and sometimes spread on to the centre; and nothing will remove them.
If the pyrogallic acid developer does not spread itself immediately across the plate, it produces lines which are as irreparable as those stains described in the previous instance. It is also very important to maintain a constant backward and forward motion during development, otherwise a series of little black points of reduced silver will attach themselves to the plate.
Hyposulphite of soda, imperfectly washed away, sometimes agglomerates after the lapse of a certain time, and then produces star-like spots of the form indicated--fig. 68, page 63. It is, therefore, of the highest importance to remove the hyposulphite by repeated washing.
It remains to say a few words on fogging, the origin of which is twofold-the first, diffused light, and the second, alkalinity of the bath.
The evil from the first cause arises generally from the inferiority of the yellow glass, which does not completely arrest the passage of the actinic rays; sometimes from the lamp or candle giving off too much white light, from a hole in the camera back, or in the camera itself, &c.
The second of these causes, alkalinity of the bath, is much more rare; it occurs generally in summer time when the weather is very warm. A few drops of acetic acid in the nitrate bath will obviate this defect.
Where crystallisable acetic acid cannot be obtained, its use may, to a certain extent, be dispensed with, by the following plan.
One thousand five hundred grains of caustic potash are dissolved in 35 ounces of distilled water, to which is added 750 grains of powdered litmus, and the blue liquid is decanted into another bottle. This bottle should be kept carefully stoppered. Having obtained some good ordinary acetic acid, called purified pyroligneous acid, and a tube divided into cubic centimetres, two cubic centimetres of the blue solution of potash are poured therein. Now add, drop by drop, some standard crystallisable arid, becoming solid when exposed to a temperature of about 40° Fahr.; and after each addition, shake the tube. A point will be reached al which the blue solution all at once becomes red; it is at this moment that the operation is completed.
A note is made of the amount of acid which was necessary to change the blue colour of the potass solution; and suppose, for example, this to have been a quarter of a cubic centimetre. Now begin afresh, by mixing the solution of litmus with twice its volume of water, and also the crystallisable acid with a similar proportion. This will allow the observation to be made more accurately as to the quantity of acid required to redden a given quantity of solution of potash. Suppose that it is finally ascertained that 10 cent. cubes of the blue solution require 1 to 1¼ cent. cube of the crystallisable acid. Now perform a similar operation with the pyroligneous acid; this being much weaker, it will probably be found that for 10 parts of the blue solution 3½ parts of this acid will required. The following equation is now made. If 1¼ parts of pure acid correspond to 3½ parts of ordinary acid, 10 of pure acid will correspond to x.
From this calculation is deduced the fact, that x is equal to 28; from which it results, that every time 10 grains of crystallisable acid are ordered in a formula, they may be replaced by 28 of ordinary acid: if 5, 14 only will be necessary; if 30, 84 will be wanted, &c. &c.
The hydrometer cannot be used to determine the strength of acetic acid, for its density bears no regular proportion to its saturating power, or, in other words, to the relative quantities of acid and water.
The pure crystallisable acid solidifies between 50° to 60° Fahr. Although the ordinary acid is only a mixture of pure acid and water, and the first solidifies at, say 56° Fahr., and the second at 32°, it does not necessarily follow that the mixture shall solidify above 32°. The pure acid mixed with three times its volume of water, should, at first sight, solidify at about 40° Fahr., but, in reality, it does not congeal until it is cooled to 36° below the freezing point of water; from which it follows, very weak acid cannot be purified by successive and fractional freezing; and it is only when more concentrated, that. this method of purification can be adopted.