May 2002 Volume 22 Number 2
![]()
![]()
May 2002 Volume 22 Number 2
The Teas diagram offers a simplified and logical way to visually locate effective solvents or solvent blends for a given polymer-resin. Published shortly after the enactment of Los Angeles County Rule 66, which limited the use of photochemically reactive solvents, its purpose was to function as a “user friendly” solvent substitution guide primarily for coating formulators.
The triangular diagram contains three coordinates that describe the solvent “power” on the basis of dispersion forces (fd), polarity (fp), and hydrogen bonding (fh), and stipulates that mixtures with resins of similar values will become solutions. A more accurate equation has been developed by Huyskens, et. al. (1985), however it requires additional data and is most easily solved via computer program rather than a visual chart.
As conservators began to implement the Teas concept of solubility, Feller (1972) illustrated that the Teas solubility coordinates for mixtures of cyclohexane, toluene and acetone give solvent mixtures with uniform spacing of fd while following a close 50 / 50 balance between fp and fh. Once solubility was found using this system of three solvents, one could then use the Teas diagram and find a suitable substitute solvent or blend that would be equally effective- i.e., using it in a manner similar to a coatings formulator seeking a Rule 66 exempt solvent.
However, it was also noted that the single parameter, generally reflective of factors contributing to polarity, is easier to decipher than the triangulation method required of the 3-axis Teas diagram. While the emphasis on polarity was what Teas was trying to refine upon, perhaps Feller’s observation gives emphasis to the materials that conservators most often confront: that is, resins that were once easily solubilized when fresh but, through oxidation, have become more polar and thus require more polar solvents.
Numerous articles have appeared in the conservation literature describing the charts strengths and weaknesses when applied to conservation. Conservators have used the Teas approach to help find suitable solvents or solvent mixtures to dissolve materials whose composition and/or age may not be completely known. Although conservation applications may often have much in common with the formulation of coatings, there are instances where the Teas concept might not accurately describe the system being treated.
Even though the Teas chart has an axis dedicated to hydrogen bonding, it only describes circumstances where opportunities for hydrogen bonding are completely random. (See Illustrations). That is, the proton-rich, i.e. proton repelling, end of the hydrogen bond and the proton-accepting end are available and accessible to an equivalent degree on both the solvent and the solute. The Teas diagram does not properly describe, for example, hydrogen bonding where only one component of the hydrogen bond (i.e. proton donor or acceptor) is located exclusively on the resin while its counterpart is on the solvent.
In such a system, mixing is discouraged because solvent-solute pairings occur and discourage randomness – the central tenet of solubility. The propensity to be attracted to opposites is not necessarily in strict defiance of the Teas concept of solubility because it is often thought of as the essence of the “like dissolves like” principle. However, while a proton donor and a proton acceptor are dissimilar, the Teas diagram cannot accurately predict the full ramifications when such a preferred pairing occurs.
If such a favored state were to occur, it is incumbent to ask what is the nature of the hydrogen bond and specifically what the new overall shape of the “molecule” is like. That is, if we think of this hydrogen bond whose lifetime is long enough (i.e., within the time frame of the conservator’s attempts at achieving solubility) we might think of it as growing in molecular size due to the solvent molecules that associate with it.1
In addition, and perhaps more important, the new terminal is likely to have altered the effective polarity and, quite possibly, its solubility. That is, once proton rich or proton lean groups on a resin have been satisfied, or sequestered, the altered resin might be soluble in solvents with different, presumably reduced, hydrogen bonding properties. By taking these considerations into account it could possibly lead to quantifying the amount of polar solvent required to act on a specific amount of material while reducing undesirable (e.g. leaching) side-effects.
It may sound optimistic to believe that certain secondary molecular associations might be leveraged to alter solubility, but this is exactly what happens to many polymers that have plasticizers added to them. Vulnerability to specific solvents is one of the trade-offs that polymer formulators are sometimes willing to accept for plasticized polymers. From the conservator’s perspective this could be an advantage. However, until this topic has been more rigorously pursued one needs to exercise caution in applying it as a treatment protocol. The Pettenkofer treatment designed to regenerate varnish is one such example of good intentions going awry. (Scmitt 1990).
There are additional ways that entropy is constrained to discourage polymer solubility. In the solution theory of macromolecules the standard model describes the polymer sub-units (formerly monomers) as approximately the same size of the solvent but attached to each other with string, representing primary covalent bonds. These “strings” pose a major impediment to a polymer’s solubility: solvent molecules have more degrees of freedom to move about whereas the movement of polymer sub-units is restricted. Hedley (1980) has pointed this out in the conservation literature, but additional aspects related to polymer structure, or morphology, are discussed below.
Solubility and optical properties are often the most important considerations for the conservator and morphology points out an interesting correlation between the two. The vast majority of conservation adhesives, consolidants and varnishes that are true solutions can be described as amorphous thermoplastics. Amorphous materials lack the three-dimensional order found in crystalline materials.
In considering this random orientation one needs to consider the space atoms in a molecule actually occupy vs. the free space, literally the free volume, around them. The random organization of amorphous materials imparts a high free volume that permits the facile penetration of a well-chosen solvent. In addition, amorphous materials cannot scatter light the way systems with 3-dimensional order can and are thus clear rather than opaque. Thus, solubility and transparency are interrelated, which, for the conservator, is a good thing.
The factors that the Teas diagram is derived from only apply to amorphous materials, it does not include the heat of fusion associated with crystalline materials (Burrel 1955). The regular structure of crystalline materials like (e.g. linear polyethylene) permits a high packing efficiency that results in a low free volume and a high density. This dense, ordered, packing impedes solubility because the voids within the crystalline domains are typically too small for solvents. Optically, the 3-dimensional structure has the potential to scatter light internally and thus appear opaque. At temperatures above the heat of fusion, i.e. the molten state, crystalline materials become amorphous liquids and are more readily solubilized.
In conservation, waxes are perhaps the most common crystalline materials that the conservator will encounter; however, based upon the experience of paintings conservators contacted, varnishes that were thought to contain wax do not present a major impediment, though one would have to conduct a more comprehensive study to verify.
However, the negligible impact wax apparently has on predicted solubility could be related to its concentration, or the varnish resin has forced a higher proportion of the wax to exist in the amorphous state. In bulk form, however, where waxes are highly crystalline they are most easily solubilized once they are molten. Whether or not the wax remains in solution upon cooling, becomes a stable suspension, or precipitates, depends upon additional factors.
To more completely understand physical changes that may influence solubility one must further consider the influence of (ambient) heat on polymer morphology. In this regard, the most important thermal transition is the glass transition, Tg,2 which is the thermal transition associated with the polymer’s amorphous phase. (A polymer that is cooled rapidly is more likely to have a higher amorphous content than if was cooled slowly and the degree to which this occurs is controlled by the polymer morphology.) The amorphous component of semi-crystalline polymers and the bulk properties of amorphous materials can densify after sufficient time, which may impede the diffusion of solvent. The extent to which this occurs will be most dependent upon the ambient temperature relative to the material’s Tg and the exposure time. These factors control a material’s thermal history and are expressed as physical aging and will be most significant for materials whose Tg is at or below the ambient temperature.
In Huyskens’ equations for determining solubility he indicates that solubility is dependent upon the crystalline / amorphous ratio for a given polymer class. McGlinchey (1993) has found subtle but consistent changes in solubility occur when naturally aged polyvinyl acetate, several decades old, were heated for short intervals above the material’s Tg. This suggests that erasing a portion of the physical aging can make small alterations in a material’s solubility properties.
Finally, Teas only considers neutral, nonionic molecules. In conservation applications one could encounter ionic species from surfactants in emulsion paints or as degradation products. One may want to solubilize them, (e.g. degraded varnish) or prevent solubilization (e.g. sensitized paint.) Whether attempting to locate solubility as one would with an aged natural resin or navigate around it, the Teas diagram will not accurately predict the influence ionic components have on the solubility. In addition to its high polarity, the ability of acetone (a polar aprotic solvent) to solvate ionic species (Streitwieser 1976) could be one additional reason why it works effectively- sometimes zealously on aged materials.
In circumstances that defy the logic of either the polarity concept or the Teas diagram it may be worthwhile to consider whether or not the factors mentioned above could be influencing solubility. Even though significant contributions examining the behavior of solvent action and identification of degradation products found in aged artists materials have been recently carried out, further studies are necessary.
Meanwhile, some basic analytical tools could help. For example, identification of functional groups via Fourier Transform Infrared Spectroscopy (FTIR) could help determine if the sample is either starved or saturated with one half of a hydrogen bond. Thermal analysis methods such as differential scanning calorimetry (DSC) or dynamic mechanical analysis (DMA) could determine the extent of physical aging that has occurred. Alternatively, hot-stage polarized micros- copy could be used to determine if (organic) crystalline components are present. Cloudiness beyond what one could ascribe to accumulated dust may indicate that the sample has become more crystalline or that additives, at one point soluble, have precipitated.
Teas himself estimated his chart to be about 80% accurate for “regular solutions” relevant to the coatings formulator, however, this value is further reduced when one includes the broadened uncertainty of what a conservator could encounter. Perhaps supporters of the Teas diagram have simply happened to work on a greater number of works that are aptly described by the Teas diagram while those that have trouble supporting it have not. Alternatively, those in the latter category may simply have acquired sufficient experience that permit their own instincts to successfully navigate towards solubility in a safe and efficient manner a majority of the time.
Whether one adopts the Teas approach or abandons it, it pays to weigh both its strengths and weaknesses. In doing so, one benefits from a more comprehensive understanding of the issues surrounding the nature of solubility and the limits of equations based on ideality. Though by focussing on the chemical and physical properties of the object itself the risks associated with conservation treatments will be minimized and research topics that enhance our understanding of their behavior will become more clearly defined.
1. The plasticizer camphor, a proton acceptor, remains hydrogen bonded to hydroxyl groups on cellulose nitrate for decades, so it is plausible to think that certain solvent-solute pairs may persist long enough for certain treatments.
2. Not to be confused with the softening point which generally occurs at 50ºC above the Tg.
Burke, John, Mark Ormsby and David Earhardt, “A Teas Refresher,” AIC News, September 2001.
Blank, Sharon, and Chris Stravroudis, “Solvents and Sensibility,” WAAC Newsletter, 11 No.2, May 1989.
Feller, Robert L., “The Relative Solvent Power Needed to Remove Various Aged Solvent Type Coatings,” IIC, Vth Congress, Lisbon, 1972.
Hedley, Gerry, “Solubility Parameters and Varnish Removal: A Survey,” The Conservator, No.4., 1980.
Huyskens, P.L., et.al. “Dissolving Power of Solvents and Solvent Blends for Polymers,” Journal of Coatings Technology, 57 No. 724 (May 1985).
McGlinchey, Chris, “The physical aging of amorphous materials,” In Saving The Twentieth Century, Canadian Conservation Institute, Ottawa, 1993.
Burrel, Harry, “Solubility Parameter,” Interchemical Review, Spring, 1955.
Schmitt, Sibylle, “Examination of Paintings Treated by Pettenkofer’s Process,” Cleaning Retouching and Coatings: Technology and Practice for Easel Paintings and Polychrome Sculpture: preprints to the Brussels Congress, 3-7 September 1990, IIC pp. 81-84.
Streitwieser, Andrew Jr. and Clayton H. Heathcock, Introduction to Organic Chemistry, MacMillan Publishing (New York: 1976) p. 143.
The Hydrogen bond (illustrated as the dashed line below) is a strong secondary force because the attraction between electropositive (+) and electronegative (-) ends is strong, and, the proton, being of such small atomic radius can get in close proximity to proton acceptors.
![]()
To insure random mixing, solvent-resin interactions must be as likely to occur as solvent-solvent and resin-resin interactions. With alcohols, the affinity for solvent-solvent interactions, which may occur, as illustrated below, could be an impediment to solvent-resin interactions.
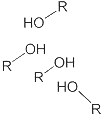
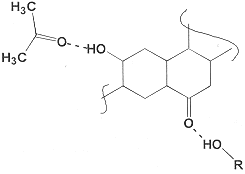
In the example below, acetone, lacking a strong proton donor, will not form strong solvent-solvent interactions but can hydrogen bond with resins containing hydroxyl groups. Similarly, alcohols will be attracted to carbonyls. In both instances, random mixing cannot be assumed.
Chris McGlinchey is the conservation scientist at The Museum of Modern Art in New York (MOMA-Queens), or QNS, until early 2005) and adjunct professor of conservation science at New York University. His address i Christopher McGlinchey, The Museum of Modern Art, 11 West 53rd Street, New York, NY 10019.