September 1998 Volume 20 Number 3
![]()
![]()
September 1998 Volume 20 Number 3
If one stands back and looks objectively at the body of technical information on the cleaning of paintings with organic solvents, there is really only a rather small core of useful material to which conservators can refer to support their understanding of the effects of these agents on paint films.
Although it was played down somewhat by some contemporary authorities such as Ruhemann, the work of Nathan Stolow, ultimately compiled in his chapter of "On Picture Varnishes and their Solvents", remains possibly the most important reference source describing the action of solvents on oil paints.1 Stolow identified two principal effects: the physical swelling of the organic binder phase caused by sorption of solvent, and leaching, i.e. the extraction of soluble, low molecular weight components of the organic binder phase. Studies of leaching of paint films by organic solvents has continued since Stolow's pioneering gas chromatographic investigations of the 1960s and 70s, and this aspect of risk in cleaning is rightly continuing as a major aspect of solvent-cleaning research.2
It is arguable whether a conservator can actually sense leaching during
the cleaning process; and, from the practical point of view, the most
useful parts of Stolow's research are his investigations of the swelling
power of various organic solvents on reference paint films. One of the key
findings was the variation in the degree of swelling of reference paint
films by organic solvents in relation to their Hildebrand solubility
parameter ![]() [ref. 1, figure 4-15]. Solvents and
solvent mixtures of intermediate polarity (i.e. those having
[ref. 1, figure 4-15]. Solvents and
solvent mixtures of intermediate polarity (i.e. those having ![]() values
in the range ca. 8.8 - 11(cal/cm3)1/2) produced the
greatest degrees of swelling. This observation is comparable with the
results of the very few other paint swelling studies that exist in the
industrial paint field, notably Browne3
and Eissler and Princen4
values
in the range ca. 8.8 - 11(cal/cm3)1/2) produced the
greatest degrees of swelling. This observation is comparable with the
results of the very few other paint swelling studies that exist in the
industrial paint field, notably Browne3
and Eissler and Princen4
The application Stolow's oil paint swelling data to actual cleaning practice owes much to later interpretations using more sophisticated treatments of the solubility parameter element, particularly that of Gerry Hedley5 and, more recently, of Stefan Michalski6. While there is no doubt that these interpretations have been valuable contributions to the technical and theoretical foundations of solvent-cleaning, it must be emphasized that they are constrained by the limitations of the original Stolow data, which are significant.
Stolow's research on paint swelling is now more than 40 years old. Considering its worth, it is rather surprising, though, that this theme has not been developed or extended further. As time has progressed the limitations of his data, and the conclusions that can be drawn from it, have become increasingly apparent, for reasons which will be described shortly. It is hoped that work now under way at the Courtauld Institute of Art in London, to measure the swelling relationships of a wide variety of paint films and solvents, will build on the foundation established by Stolow through examination of a much broader range of paint film types and solvents. This article provides an opportunity to present some of the results of preliminary experiments.
Ever astute, Ruhemann spotted the constraints on interpretation of Stolow's research deriving from the nature of the paint films which formed the subject of his investigations. The essential point is that Stolow's swelling data comes from experiments on paint films composed of lead white in linseed stand oil, all younger than 25 years old. Such films, we know now, will be significantly different in chemical character to those of old paintings. Both the comparatively young age and the choice of stand oil as binder [dried films of stand oil are known to be considerably less oxygenated than those of blown or boiled oils or simple oils dried normally in air] suggest that Stolow's data significantly under-estimates the response of real, old paint films to solvents at the more polar end of the spectrum, particularly to those that are strongly dipolar and/or engage in hydrogen bonding.
Practical wisdom suggests that, in many cases, paint films can be
vulnerable to solvents and solvent mixtures over a much broader
range than indicated by the Hedley/Stolow "peak swelling
region", particularly on the polar side. Ethanol, for example
is a rather low-swelling solvent according to Stolow's data, but in
practical restoration this solvent is perhaps more rightly regarded
as potentially having a strong effect on paint. It might be expected
that the solubility regions of old oil paint films are shifted
towards the direction of higher polarity. Some slight indication of
this shift is indicated in the swelling data of Stolow for paint
films of different age, with maximal swelling moving to slightly
higher values of ![]() for the older films[ref. 1 fig. 4-15].
for the older films[ref. 1 fig. 4-15].
It seems clear now that we need to up-date our view of the internal chemistry of aged paint films from the general model which is implicit in the work of Stolow. Stolow's model essentially treats dried oil films as a three-dimensional network polyester polymer based on fatty acid triglycerides, and containing a low molecular weight mobile phase of unpolymerised triglyceride units and breakdown oxidation scission products. Further oxidative degradation on ageing is seen as a continued source of solvent-extractable material.
Cohesion of the paint film derives essentially from the cross-linked oil matrix which, although insoluble, can be swollen to varying degrees by organic solvents. In the swollen condition, the low molecular weight compounds, can be extracted (leached) by the solvent, leaving the film compacted, stiffer and more vulnerable to physical degradation. Implicit in Stolow's description of paint/solvent interaction is the notion that the solvent interacts primarily only with the organic component by solvation processes, so causing swelling and softening of the medium, with consequent lessening of its capacity to bind the pigment. It is suggested, now, that this model is appropriate really only for relatively young oil films typified by those which formed the subject of Stolow's tests.
Fortunately, some of our analytical chemists have now finally directed their attention to ageing and deterioration processes in artists' paints, rather than concentrating on purely archaeometric identifications, and information to support a broader model for the chemistry of oil paint ageing is now beginning to emerge. The studies of Schilling7 and of Boon and his colleagues in the MOLART project in the Netherlands are particularly important in this respect. In the light of recent analytical results from within MOLART, a new model for the chemistry of paint ageing has been postulated. Professor Boon8 described a general scheme for different developmental stages in both oil and egg tempera paint films and, more recently, J.D.J. van den Berg has presented analytical data to support Boon's general hypothesis.9,10
A full description of the model is not appropriate here, and the reader is directed to the cited publications for a detailed explanation. However, it is worth noting that the new MOLART model suggests that continued ageing of paint films will, by oxidation and/or hydrolysis (i.e. de-esterification of glyceride linkages), lead to increased polarity in the paint film, and to the increasing anionic functionality of the paint binder. The formation of oxygenated functional groups (ethers, peroxy cross-links, hydroxyls, carbonyls, etc.) into both the stationary and mobile phases will inevitably increase sensitivity to polar and hydrogen-bonding solvents. Since metal ion/medium interactions would contribute strongly to the overall cohesion of the paint film, these liquids might also disrupt paint film cohesion and pigment binding by interfering with the carboxylate/metal ion interaction, in addition to any solvation or swelling effect on the organic binder. Ion-dipole and ionic interactions between solvent and paint may become increasingly important in governing the response of paint films to cleaning liquids as they increase in age or state of deterioration.
In summary, our understanding of the solvent-swelling behavior of old oil paints is not well-developed; and the same can be said for paint films based on binding media other than linseed (stand) oil, such as oil/resin paints and egg tempera. Also, it is now well-established that the nature of pigment in the paint film influences the pattern of drying and deterioration; thus pigmentation is expected to have considerable effect on solvent-swelling response. Lead white paint films are generally very low swellers, and other pigments, like carbon blacks or red lakes, might be expected to be rather more sensitive to solvents.
It is the influence of these factors, therefore, which our experiments intend to address. We hope to examine the swelling response of a wide range of paint film types to a range of solvents that represent both typical cleaning liquids and distinctive solvency characteristics. Furthermore, oil and resin films are known to become increasingly acidic on ageing, and it is likely that acid/base interactions will have an increased influence on the solubility and swelling behavior of the organic binder phase of old paintings. In addition to testing neutral organic solvents, we will also be conducting experiments on the swelling action of cleaning liquids that are alkaline, for example, solutions of ammonium hydroxide or triethanolamine in water.
The hope is that the work will additionally indicate something of the internal cohesive chemistry of old paint films, and thus be complementary to instrumental analytical investigations of this subject. To do this we will need to describe solubility behavior in rather more sophisticated ways than we do at present. As has been discussed previously within this Newsletter 11, the solubility parameter systems currently used within conservation for describing the properties of organic solvents and their interactions with solutes (i.e. paint and varnish) are crucially limited in their treatment of polar aspects, particularly hydrogen bonding and acidity/basicity.
Recent developments in systems for specification of solubility behavior have been reviewed recently.12 An outcome of this has been the proposal of a new composite solvent specification system based on recent developments in solvency theory within the field of liquid chromatography, which should be capable of better description of paint/solvent interactions. It is proposed that this new system (which describes contributions to solvent properties from each of dispersion forces, dipolar interactions, hydrogen-bonding acidity and hydrogen-bonding basicity) will provide an improved model for presentation of swelling data compared to existing schemes.
New studies on the swelling of artists' paints There are surprisingly few references in the literature that relate to the swelling of paint films by organic solvents or aqueous liquids. Michalski has collated data from the most relevant works, namely Stolow, Browne, and Eissler & Princen. Plotted in graphic form, this gives the general picture of paint swelling in relation to solvent polarity that is illustrated in Figure 1.
Here polarity is represented, inversely, by the Teas fd solubility parameter, but it is likely that any indicator of polarity for the x-axis in this graph would give qualitatively similar results. This clearly indicates the differing capacities of the various solvents to bring about swelling of oil paints; or at least the young oil paints which have so far been investigated. Not surprisingly, it is the chlorinated solvents which feature in many paint strippers that cause the strongest swelling effect. Maximal swelling is caused by solvents with an fd value of 67-68, and solvents in this region might be expected to involve the highest level of risk in cleaning.
As far as can be established, Stolow's is the only research that has attempted to determine swelling by measurement of changes in paint film thickness, for which a dedicated instrument was designed and built.13 The more usual approaches to measuring the solvent-induced swelling of paint or polymer films involve weight gain and change in volume of samples (as in Browne), or change in perimeter (as in Eissler & Princen). The experimental procedure of Eissler & Princen, which derives from Brunt14 is interesting for its simplicity: small triangular paint fragments were immersed in various solvents, and swelling was determined by measuring at intervals the perimeter of the samples by means of a travelling microscope. Whilst being generally effective, this method is highly labor intensive and subject to a fair degree of error, partly related to curvature in the sample edges during swelling.
Our approach has been to take the general method of Eissler & Princen - i.e. to monitor continuously the dimensions of paint samples whilst they are immersed in solvent ­ but to improve on the measurement aspect, taking advantage of electronic image capture and computer-based image analysis as the means of achieving accurate readings of sample dimensions. The full experimental procedure is in the process of refinement and, we hope, will be published in detail in the near future.
In essence, though, the method involves examining particle fragments of paint ranging in area from ~1- 15 mm2 at low-power magnification (10-40X) over time, from the moment they are immersed in solvent. Digital images of the samples, obtained under constant conditions, are captured at regular intervals over time periods from t=0, typically extending to 30-60 minutes.
The constant conditions of image capture and calibration of the images as a group ensure a high degree of internal reliability in the data. The images thus captured at varying stages of swelling are processed electronically using two software programs: Adobe Photoshop, in which the images are adjusted in brightness and contrast and sharpened, and Graftek Optilab V. 2.6.1 in which they are calibrated and then thresholded to render each sample as a discrete particle.
Particle analysis functions within the Optilab software then allow the dimensions and conformation of each particle/sample to be described in terms of more than 20 different parameters. Rather than particle perimeter which was used by Eissler & Princen, particle area has been found to be the more accurate descriptor of paint particle swelling. Perimeter measurements were found to be highly sensitive to pixelation generated during image capture and processing. Since solvent volume is greatly in excess, several paint samples can be monitored simultaneously, thus improving statistical validity. Alternatively, paint samples of different compositions can be measured in a single experiment. Either way, the method provides an efficient means of generating swelling measurements on a reasonably large sample population. The parameters measured are effectively the rate of swelling as determined by the time to maximal or equilibrium swelling, and the actual magnitude of maximum swelling, expressed as a percentage increase on original area.
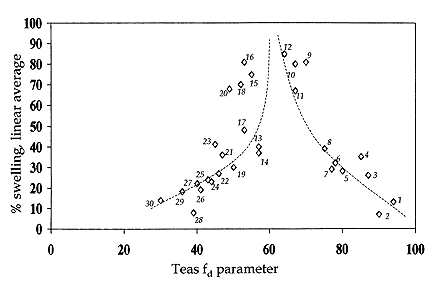
| Figure 1. Variation in oil (paint) swelling as a function of Teas fd fractional solubility parameter, based on data compiled by Michalski6. |
| 1. | cyclohexane | 11. | dioxane | 21. | propanone (acetone) |
| 2. | mineral spirits | 12. | trichloromethane/ethanol 9:1 | 22. | pentan-1-ol (n-pentyl alcohol) |
| 3. | ethyl benzene | 13. | pentan-2-one* (methly propyl ketone) | 23. | diacetone alcohol |
| 4. | tetrachloromethane (carbon tetrachloride) | 14. | propylethanoate | 24. | butan-2-ol* (sec-butyl alcohol) |
| 5. | toluene | 15. | cyclohexanone | 25. | butan-1-ol (n-butyl alcohol) |
| 6. | benzene | 16. | cyclohexanone/ethanol 9:1 | 26. | propan-2-ol (isopropyl alcohol) |
| 7. | turpentine | 17. | butanone (methyl ethyl ketone) | 27. | propan-1-ol (n-propyl alcohol) |
| 8. | dipentene | 18. | ethanol/turpentine 6:4 | 28. | acetonitrile |
| 9. | benzene/ethanol 8:2 | 19. | 2-ethylhexanol | 29. | ethanol |
| 10. | trichloromethane | 20. | ethanol/toluene 7:3 | 30. | methanol |
| *fd values for solvents marked with an asterisk are estimates based on known data for solvents of similar chemical functionality. |
To test the method, a preliminary series of swelling experiments have been conducted on paint samples from an abundant available supply. These are unsupported samples, prepared in 1993 by the doctor-blade method, of burnt umber paints from the Winsor & Newton Artists'Oil Colour range, thermally aged by heating to 80�C for 12 days. Film thicknesses are generally in the range of 220-330µm. It is appreciated that these samples may not be typical of paints found in old pictures: they are still quite "young" in character, being still pliable, glossy and tough. They do, however, provide good comparison with Stolow's young lead white/stand oil paint films and their selection as initial sample subjects was in part driven by the intention to reproduce the general pattern of Stolow's results but using a different experimental approach.
Figure 2 illustrates the different rates and magnitudes of
swelling of the thermally-aged burnt umber paint films caused by
various common alcohol and ketone solvents. As might be expected
from what is known from Stolow's experiments, the smaller homologues
of a series cause more rapid swelling, results which are explicable
in terms of molecular volumes and diffusion coefficients. Thus
acetone (propanone) swells slightly faster than butanone, both
reaching maximal swelling within 10 minutes. The much larger
molecule, cyclohexanone, by contrast is a slow diffuser and takes
30-40 minutes to reach maximum swelling. The alcohols are generally
slower diffusers/swellers than their corresponding ketones;
nevertheless, methanol and ethanol both reach maximum swelling quite
quickly, in 13 and 15 minutes respectively. The much more bulky
tert-butyl alcohol  (2-methylpropan-2-ol) is a very much slower
diffuser/sweller, and has still not reached maximum swelling after
40 minutes. The times to maximal swelling are comparable with the
rates measured by Stolow if one takes into account the differences
in paint film thickness between his and these experiments. The
swelling curves for acetone, butanone, methanol and ethanol all here
show slight contractions after the maximum has been reached
­ also observed by Stolow ­ a phenomenon which is
almost certainly due to loss of soluble material (leaching) from the
swollen paint binder. An interesting observation is that within the
ketone series the magnitude of the value of maximum swelling
increases as the molecular size of the solvent increases: maximum
swelling for cyclohexanone is 25.6% compared to 19.8% for acetone.
The pattern within the alcohol series is different, and swelling
power decreases with increasing molecular volume. Indeed, for this
particular paint film the higher (C3 - C4) alcohols have
comparatively low swelling power.
(2-methylpropan-2-ol) is a very much slower
diffuser/sweller, and has still not reached maximum swelling after
40 minutes. The times to maximal swelling are comparable with the
rates measured by Stolow if one takes into account the differences
in paint film thickness between his and these experiments. The
swelling curves for acetone, butanone, methanol and ethanol all here
show slight contractions after the maximum has been reached
­ also observed by Stolow ­ a phenomenon which is
almost certainly due to loss of soluble material (leaching) from the
swollen paint binder. An interesting observation is that within the
ketone series the magnitude of the value of maximum swelling
increases as the molecular size of the solvent increases: maximum
swelling for cyclohexanone is 25.6% compared to 19.8% for acetone.
The pattern within the alcohol series is different, and swelling
power decreases with increasing molecular volume. Indeed, for this
particular paint film the higher (C3 - C4) alcohols have
comparatively low swelling power.
Table 1 is a compilation of data on the maximum swelling of the thermally-aged burnt umber/linseed oil paint films for a range of solvents, together with some solvent mixtures and alkaline solutions. The thickness of the paint films examined in each case is given in column 4. Values of Teas fractional dispersion force parameters (fd) are also quoted as arbitrary indicators of polarity.
| Table 1. Values for maximal swelling of burnt umber linseed oil films, aged 12 days @80�C,for various solvents. |
| No. | Solvent | Teas fd parameter | Paint film thickness (µm)z | Average area swelling % |
| 1. | perfluorodecalin | 100 | 230 | 0.7 |
| 2. | iso-octane | 100 | 330 | -4.75 |
| 3. | white spirits | 90 | 230 | 7.52 |
| 4. | tetrachloromethane | 85 | 330 | 1.5 |
| 5. | ethylbenzene | 87 | 320 | 9.7 |
| 6. | dibutyl ether | 70* | 230 | 10.9 |
| 7. | dioxane | 67 | 220 | 23.5 |
| 8. | amyl acetate | 62 | 370 | 11.6 |
| 9. | cyclohexanone | 55 | 220 | 25.6 |
| 10. | dichloromethane | 59 | ­ | 38.8 |
| 11. | butanone | 53 | 310 | 20.3 |
| 12. | IMS/iso-octane | 68 | 340 | 7.3 |
| 13. | acetone | 47 | 330 | 19.8 |
| 14. | N-methylpyrrolidone | 48 | 300 | 34.7 |
| 15. | tert-butanol | 44* | 230 | 6.9 |
| 16. | DMSO | 41 | 230 | 22.2 |
| 17. | propan-2-ol | 38 | 320 | 5.1 |
| 18. | butan-1-ol | 43 | 330 | 6.8 |
| 19. | methoxypropanol | 42 | 300 | 14.3 |
| 20. | ethanol | 36 | 300 | 15.6 |
| 21. | IMS | 36 | 360 | 9.5 |
| 22. | acetone/water 1:1 | 32.5 | 230 | 18.5 |
| 23. | methanol | 30 | 310 | 17.4 |
| 24. | trifluoroethanol | ­ | 220 | 23.0 |
| 25. | X | 52* | 320-370 | 2.3 |
| 26. | triethanolamine solution pH 9.7 | n/a | 230 | 37.9 |
| 27. | ammonium hydroxide solution pH 11.2 | n/a | 230 | 52.5 |
| *values marked with an asterisk are estimated values based on known data for compounds of similar chemical functionality. |
Figure 3 shows the swelling data of Table 1 plotted against Teas fd for comparison with the extant data as compiled by Michalski (Figure 1). For the thermally-aged burnt umber films, the general pattern of swelling in relation to Teas fd is similar to that observed in previous studies, with solvents in the fd range 40-60 mostly producing the highest levels of swelling. The magnitudes of linear swelling compare well with those measured by Browne and by Eissler & Princen, with the strongest-swelling solvents causing >30% increases in dimension. As Stolow observed, strongly apolar solvents, such as the saturated hydrocarbons, cause very little paint swelling. In fact, here, iso-octane caused some slight shrinkage in the sample during immersion, for which the reasons are not clear, but may relate to displacement of absorbed atmospheric water.

Figure 3. Maximum swelling of thermally-aged burnt umber oil paint as a function of Teas fd fractional solubility parameter. See Table 1 for key to number representing solvents.
It is, however, at the opposite end of the polarity scale that the results show significant divergence from the findings of Stolow. As described earlier, according to Stolow's results the lower alcohols are low swellers: methanol causing swelling of the same order of magnitude as the aliphatic hydrocarbons. The present results give a rather different picture. Although C3 and C4 alcohols are low-to-moderate swellers, ethanol and methanol have an appreciable swelling action on the paint films which are the subject of these tests, with methanol giving the highest swelling (17.4%) of the homologous series of alcohols. Whether this is evidence to support the hypothesis presented earlier, that paint's response to polar solvents increases with increasing age/deterioration, remains to be seen. This matter will be an important focus of further experiments.
Another aspect in which the body of swelling data presented in Figure 3 differs from the extant data is in the consistency of action in relation to dispersion force parameter. The pattern of response is altogether less coherent than in Figure 1. A number of solvents and solvent mixtures having fd in the range 40-60 actually produce quite low levels of swelling. This combination of properties ­ intermediate polarity and low paint swelling ­ may be useful for limiting risk during varnish removal. One solvent in this polarity range, in particular, repeatedly caused unexpectedly low levels of swelling, ca.2.5%, in the burnt umber test paint. This solvent, no. 25 in Figure 3, is a difunctional, oxygenated solvent not presently used to any significant extent in conservation; but it is quite capable of dissolving moderately aged natural resin varnishes and is reasonably safe. For the present it must remain anonymous, pending further experiments of its action on other paint film types. The possibility, however, that there exist solvents having reasonable polarity but which generate such low levels of swelling in oil paints is of considerable interest in the context of refining cleaning practices.
Natural resin varnishes are known to become increasingly acidic as they age and oxidize. Conservators, quite legitimately, often look to alkaline cleaning liquids ­ typically, aqueous solutions of ammonium hydroxide ­ as a means of removing such coatings from paintings. The problem is that there is also evidence that oil paints become more acidic on ageing. Also, even before any risk of hydrolytic breakdown of the paint medium, raised pH levels have been shown to promote the swelling of oil paints in aqueous solutions.15
We might expect therefore, that swelling of paints in aqueous solutions is quite strongly pH dependent; and also that liquids which combine alkalinity and organic solvent activity, such as formulations including aqueous solutions of organic bases like triethanolamine, have especially strong effects on oil paints. Some preliminary evidence for the magnitude of these effects is given by the data in the last two rows of Table 1. Admittedly, in relation to usual cleaning practices these solutions are towards the "strong" end of the range, but the swelling caused by them (>35%) gives some idea of the magnitude of effect they may ultimately have on paint films of this type; comparable with the most strongly-swelling neutral organic solvents.
Another phenomenon identified by Stolow was the reduction in the magnitude of swelling of a paint film in a given solvent as a consequence of continued ageing, although he asserted that the general relationships among solvents is maintained. This may well be true for the pre-leached lead white/stand oil films, 25 years old or less examined by Stolow, but whether this holds for seriously old paint films is another matter. Stolow's data on this [ref. 1, Fig. 4-16] does indeed show a decline, over the period between 1 year and 25 years, in the magnitude of swelling of these films in acetone and butanone; but for methanol and ethanol, this effect is not so evident.
We would certainly expect the magnitude of swelling of a paint film to decrease as it ages over the early part of its life, not least because of increased cross-link density; but also because of a general loss of mass from the organic phase through volatilization of scission products. Both Schilling and Boon et al. have reported significant losses of carbon from oil binders. It remains to be seen how far this reduction in paint swelling capacity might proceed with continued ageing, and the levels of swelling that solvents may generate in typical old paint films. A small number of experiments have so far been performed on paint films other than the thermally-aged burnt umber samples described earlier.
Table 2 shows the magnitude of maximum swelling in acetone of a number of different paint films. A detailed analysis of these preliminary results is not appropriate here, but some general observations can be made. Firstly, the nature of pigmentation does have a strong effect on the actual degree of swelling. Oil paint films containing a high proportion of lead white (nos. 2,5 and 10 in Table 2) are generally quite low in their swelling response. Ageing, whether by light or heat, reduces the overall magnitude of swelling quite considerably. The historical materials from paintings (nos. 10 & 11) showed very low levels of swelling in acetone, implying that, to measure this property successfully in old paint films, will probably require increased sensitivity in the image acquisition set-up. The artificially-aged egg tempera paint film showed a very low level of swelling in acetone. The sensitivity of egg tempera paints to other, especially aqueous, liquids will be tested in due course.
| Table 2. Degree of swelling of various paint films due to immersion in acetone. |
| No. | Paint film | Maximum change in area (%) |
| 1. | Burnt umber oil paint, thermally aged 12 days at 80�C | 19.8 |
| 2. | Lead white oil paint, thermally aged 12 days at 80�C | 8.4 |
| 3. | Yellow ochre/lead white oil (#1), naturally aged in the dark for ~7 years | 14.2 |
| 4. | Yellow ochre/lead white oil (#1), light aged by 2 years exposure to uv-filtered daylight | 10.8 |
| 5. | Yellow ochre/lead white oil paint (#3), light aged by 2 years exposure to uv-filtered daylight | 3.25 |
| 6. | Viridian/flake white (#1) oil paint, naturally aged in the dark for ~7 years | 14.2 |
| 7. | Viridian/flake white (#1) oil paint, light aged by 2 years exposure to uv-filtered daylight | 3.8 |
| 8. | Viridian/flake white (#2) oil paint, naturally aged in the dark for ~7 years | 4.25 |
| 9. | Viridian/flake white (#2) oil paint, light aged by 2 years exposure to uv-filtered daylight | 3.9 |
| 10. | Priming from 19th century loose-lining canvas | 2.1 |
| 11. | Red paint (A) from an 18th century painting | 1.3 |
| 12. | Lead white egg tempera, light aged 413 hours in Fade-o-meter and thermally aged 504 hours@70�C | 0.5 |
These pilot tests replicate Stolow's general pattern of results reasonably faithfully, suggesting that the experimental method of measuring lateral, in-plane swelling by means of video microscopy and computer image analysis is a viable alternative to thickness or volume measurements. There is some evidence, though, to suggest greater swelling power in certain polar solvents than previously indicated. The next stage of experiments will focus on the solvent-swelling response of a variety of different paint films, with the intention of providing a broad body of data that will help to classify paint-solvent interactions.
This work was started whilst the author was on secondment to the MOLART project (Molecular Aspects of Ageing in Painted Works of Art) which is a "Prioriteit"programme funded directly by the Dutch Organization for Scientific Research (NWO). The author is indebted to colleagues at the FOM Institute for assistance and for fruitful discussions on paint chemistry and ageing, especially Prof. Jaap Boon and Jorrit van den Berg. Thanks must also go to Tom Bilson of the Courtauld Institute of Art for continuing assistance with the digital imaging and analysis.
1. Feller, R. L., Stolow, N., and Jones, E. H., On picture varnishes and their solvents, revised and enlarged edition, National Gallery of Art, Washington DC, 1985
2. For example, the work of Ken Sutherland, Culpeper Fellow, Scientific Research Division, National Gallery of Art, Washington DC
3. Browne, F.L., Swelling of oil paints in water VIII Swelling of linseed oil paints in water and organic liquids, Forest Products Journal, 6 (1956) 312-318
4. Eissler R.L. and Princen, L.H., Effect of some pigments on the tensile and swelling properties of linseed oil films, J. Paint Technology 40 No. 518 (1968) 105-111
5. Hedley, G., Solubility parameters and varnish removal: a survey, The Conservator 4 (1980) 12-18
6. Michalski, S., A physical model of the cleaning of oil paint, in preprints to International Institute for Conservation Congress, Cleaning, Retouching, Coatings, Brussels 1990, 85-92
7. Schilling, M.R., Khanjian, H.R. and Carson, D.M., Fatty acid and glycerol content of lipids; effects of ageing and solvent extraction on the composition of paints, Techne 5 (1997) 71-78
8. Boon, J.J., Peulve, S.L., van den Brink, O.F., Duursma, M.C., O'Connor, P., Rainford, D., Molecular Aspects of mobile and stationery phases in ageing tempera and oil paint films, post-prints to conference on Early Italian Paintings: Techniques and Analysis (SRAL, Maastricht, 1997) 35-56
9. van den Berg, J.D.J , van den Berg, K.J., and BoonJ.J., GC/MS analysis of fractions of cured and aged drying oil paint, poster, ICMS Conference, Tampere, Finland (1997)
10. van den Berg, J.D.J., Boon, J.J., and Phenix, A., Analytical chemistry of oil paint: a revised chemical model of aged oil paint relevant to the cleaning of paintings, in preprints to colloquium Beobachtungen zur Gemäldeoberfläche und Möglichkeiten ihrer Behandlung, Schule für Gestaltung, Bern, Switzerland 13-14 March 1998
11. Blank, S and Stavroudis C., Solvents and Sensibility, WAAC Newsletter 11, No. 2 (1989) 2-10
12. Phenix, A., Solubility parameters and the cleaning of paintings: an update and review, Submitted to Zeitschrift für Kunsttechnologie und Konservierung, Dec. 1997
13. Stolow, N., A modified apparatus for measuring the swelling of polymer films in solvents, J. Scientific Instruments 31 (1954) 416-420
14. Brunt, N.A., J. Oil and Colour Chemistsí Assoc. 47 (1964) 31
15. Eissler, R.L. and Princen, L.H., Swelling of linseed oil in acid and alkaline environments. Journal of Paint Technology, 42, No. 542 (1970) 155-158