Volume 18, Number 2 .... May 1996
![]()
![]()
Volume 18, Number 2 .... May 1996
This bibliography is an especially important and ground-breaking AATA compilation. The project grew out of a scientific research project, as well as a training course, at the Getty Conservation Institute to study and disseminate information on the consolidation of ethnographic painted objects. Research into the problem began with a literature search, which revealed that there was much relevant information available from the perspectives of numerous related disciplines. In considering the interdisciplinary nature of the problem, the Editors proposed a special supplement to AATA, since material in areas such as anthropology, ethnobotany, art history (with an emphasis on artists' notes, interviews, or descriptions of materials and techniques), and coatings science had not been previously abstracted in the regular volumes of AATA.
Like earlier AATA supplements, this bibliography is presented as a focused view of a unique problem, that of consolidating paint containing little or no binder, often, but not exclusively, found on ethnographic objects. The aims of this bibliography are two-fold:
This supplement provides other original material. The Editors' Introduction serves as a useful guide to the development of each of the four major subject areas covered: "History and Technology," "Analysis and Examination," "Properties," and "Treatment." A commentary on the material and its significance is provided. The organization of the abstracts is also unique to this supplement; within each major chapter the abstracts are grouped first according to the original date of publication, then by author. The Editors proposed this scheme as a visual way to scan the chronological development of the literature in each of the four major areas. Each abstract, whether previously published or not, was reindexed for the supplemental subject index.
A glossary, containing over 200 unique concepts, has been compiled by the Editors as a guide to the ever-changing language of paint technology. In this compilation, every attempt was made to use consistent language, despite the fact that the abstracts cover literature published during the course of two centuries. This glossary presents the terms and their commonly accepted definitions, and sources for those definitions, as used in this work.
Jessica Brown, Managing EditorThe literature references assembled in this supplemental bibliography are relevant to the conservation of painted surfaces that are matte in appearance and that require stabilization to prevent loss of material. Because the problem is so widespread amongst ethnographic collections, special emphasis is given to these objects. Preventive conservation, such as environmental control and physical support and protective systems, although normally the first line of defense, will not be addressed, as it has been extensively dealt with in the literature. This bibliography focuses on the factors affecting interventive conservation where stabilization is achieved through the introduction of a consolidant.
In a preliminary review of the literature and in wide-ranging discussions with conservators about the problems and treatment of matte paint, a number of issues stood out that had a bearing on the direction this project took. Firstly, there appeared to be a considerable discrepancy between the knowledge and skills of conservators relating to the stabilization of matte paint and the rather small amount of information that has actually been published. There is a wide variety of methods and materials that are routinely and successfully used to consolidate unstable matte paint. While many of these treatments achieve the desired outcome, not all have been developed with a complete understanding of the deterioration and treatment mechanisms involved; nor is there widespread awareness of them in the conservation community. In addition, although there are many excellent studies in the scientific literature of the physical, chemical, and optical phenomena associated with the properties of paint and potential treatment materials, there are still very few published case histories where theoretical concepts have been translated into practical trials or actual treatments of objects. This shortage of applied literature makes it difficult to construct basic models on which to develop treatment strategies for particular problems. It also inhibits critical appraisal of the existing literature and increases the risk of reinventing treatment systems each time the same problem is encountered. It is hoped that this introductory review to the present bibliography will prove useful in bridging this gap, making the range of literature cited more easily accessible than was previously the case.
Accordingly, this introductory review has several objectives. The first is to provide a guide to the range of literature included in the bibliography, pointing out references of special interest, particularly with respect to the first three chapters, "History and Technology," "Analysis," and "Properties," which may be outside of the mainstream literature commonly used by conservators, such as literature from the fields of anthropology, ethnobiology, and coatings science.
Other aims are to point out those articles, among the wide range of references included in this supplement, which are particular landmarks in their field; those which are most relevant to conservation; and the reasons for including some apparently peripheral articles whose relevance might not be readily apparent. A further aim is to make the literature abstracted in this supplement more accessible through a discussion of the interrelationship of the four chapters. This is most apparent in the last chapter, "Treatment," where, in contrast to the earlier chapters, the coverage has been as inclusive as possible. In the text, illustrations, and schema, a general framework is suggested through which to pursue appropriate responses to the wide variety of paint problems and treatment requirements.
It should be pointed out that, in order to publish this bibliography in a single volume, this work does not include a number of subjects of vital concern to conservators which are also relevant to the treatment of matte paint. Specifically, it does not directly address the ethical issues of treatment; the relationship of the substrate to the deterioration of the paint; the ways that the composition, structure, or behavior of support materials may influence the choice of treatment methods and materials; and, environmentally-induced deteriora- tion of paint and treatment materials and preventive conservation measures. Each of these important subjects would deserve an equal, if not greater, number of abstracts as included in this supplement to provide more than a cursory coverage. An exception is made in regard to the discussion of the stability of conservation treatment materials in the introductory text, because the treatment methods could not be meaningfully discussed without comment on the choice of materials.
The first chapter of the bibliography traces the history, technology, and use of matte paint applied to objects in various cultural and historical contexts. Because confirmation of observations or conclusions relating to the technology are often subject to verification, methods for determining or verifying the composition of paint, its condition, and causes of deterioration are included in the second chapter. The third chapter, on physical and optical properties of high pigment volume paint, explains why matte, porous paint is characteristically light in color and prone to instability. The fourth and final chapter on treatment focuses primarily on painted ethnographic objects, the materials and systems used in their treatment, and whether they were effective. A large number of articles that describe treatments for painted archaeological, historical, and modern or contemporary art objects are also included, where the methods or materials have application for ethnographic objects, or where the treatment approach provides a useful model for the study and treatment of matte paint in general. The citations are arranged chronologically and, within each year, alphabetically by the authors' names.
Paints with low amounts of binder have a high ratio of pigment volume to binder volume, a condition referred to in the coatings literature as a high pigment volume concentration (high PVC). These paints may have poor cohesive and adhesive properties. They normally have a matte appearance and are often in a powdery, friable, and flaking condition. Their treatment requirements differ from paints containing higher proportions of binder (such as commonly encountered linseed oil or acrylic paints) in that consolidants are easily absorbed into the paint and fill voids between the pigment particles. However, although cohesion of the paint and adhesion to the substrate are promoted by added amounts of resin, filling void spaces between pigment particles may cause changes in the appearance of the paint by a treatment that is practically irreversible. The aim therefore, when consolidating porous, matte paint is to use a consolidation system that distributes the consolidant in a manner that minimizes changes in appearance, introduces the minimum quantity necessary to achieve effective cohesion of the paint and adhesion of the paint to the substrate, and is compatible with the paint and support materials in the long term.
Some physical and optical properties of matte porous paint are considered in this chapter in order to lay a broad foundation for the discussion of possible reasons for successful or unsuccessful treatment strategies presented in Chapter 4, "Treatment."
Applying a consolidant to the surface of a powdering matte paint may cause darkening or an increase in gloss resulting in a marked change in appearance. A noticeable "tide line" (a dark outline defining the extent to which the consolidant solution has spread) may appear if the surface is not fully covered. An adhesive solution, applied at the interface of a substrate and a porous flake, may wick into the flake resulting in similar changes in appearance. Two of the major difficulties encountered in the consolidation of this type of paint are: 1) imparting sufficient consolidation to the paint and adhesion of the paint to the substrate; and, 2) determining how to measure or quantify the effect that the application of different materials by various methods has on the physical and optical properties.
Changes in lightness and chroma are often due to changes in the pigment volume concentration (PVC), defined as the ratio of the pigment volume to the pigment and binder volume:
pvc=(pigment volume)/(pigment volume + binder volume)
The coatings industry has long been aware of the importance of an understanding of PVC, especially the property of critical pigment volume concentration (CPVC), because PVC affects the manufacture, application, performance, and appearance of coatings (Asbeck 1992). It is just as imperative for conservators working with painted surfaces as for coatings manufacturers to be aware of what PVC and CPVC are and how they affect paint properties. For the conservator, this understanding is an important consideration in the selection of suitable treatments for high PVC coatings.
Since the late 1920s (Stieg 1973), it has been recognized by the coatings industry that the formulation of paint needed to be approached on the basis of volume relationships rather than weight relationships. The reason for this is illustrated in Figure 1, the pigment volume "ladder'' (Asbeck 1992). The ladder shown is a schematic of a paint system where all pigments have the same size and refractive index. It illustrates a 10% increase in each "step" from 0% pigment, totally comprised of binder, to 100% pigment (highest PVC). As the percentage of binder is decreased, the void space between pigment particles increases past the CPVC.
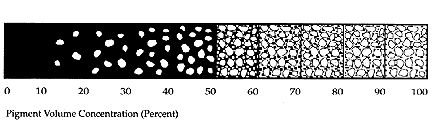
Figure 1. The Pigment Volume Concentration Ladder (after Asbeck)
The CPVC is the point when all the air between pigment particles has been replaced with binder. In practice, CPVC must be experimentally determined for each pigment and binder system; CPVC generally falls in between 30% and 65% PVC (Feller and Kunz 1981).
Below the CPVC, with excess binder, the surface becomes smoother and glossier. Many coating properties change radically at CPVC, because this is the idealized point where the coating changes from being a porous system to a solid system. This can be demonstrated by plotting properties, such as gloss and permeability, against the PVC ladder, where representative curves are illustrated in Figure 2.
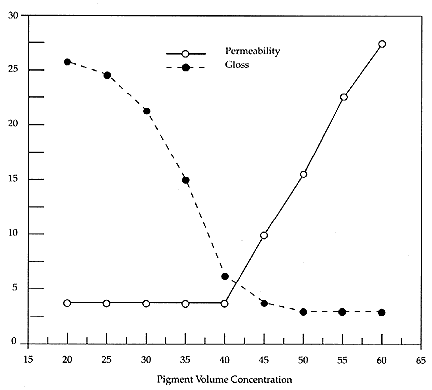
Figure 2. Representative Graphs of Changes in some paint properties
plotted versus the PVC (after Asbeck and Van Loo 1949)
Both the physical and optical properties of a paint depend upon the PVC. A matte, porous paint is at a PVC above the CPVC. Because little binder is present, extendability and strength decrease at higher PVCs. The lack of physical integrity is one reason why porous paint often needs consolidation.
The general reason why darkening occurs is due to the way diffusely reflected light is perceived. Below the CPVC, pigment particles protrude minimally above the binder, and in the case of a smooth surface the specularly reflected light will be perceived as glossy or shiny. When the amount of binder is reduced and pigment particles increasingly protrude above the surface of the binder, the surface becomes rougher (less mirror-like) and the light hitting the surface is more scattered or diffusely reflected. The scattered light will be additively mixed as the "white light" of the illuminant to the reflectance spectrum of the object surface, and the net effect will be the perception of a lighter color.
Thus, differences in the surface structures of materials must be avoided if colors are to match, as in the case of before-and-after consolidation treatment. This problem has long been recognized in the coatings industry, where two colors which appear identical at one angle, but which no longer match if the viewing angle is changed, are called "geometric metamers." The diagram of geometric metamerism reproduced in Figure 3, showing the effect of the amount of pigment protruding from a film, was originally prepared (Johnston 1967) to emphasize the critical effect PVC has on the appearance of matte paint.
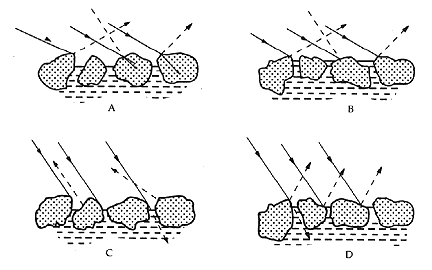
Figure 3. Geometric metamerism (after Johnson 1967). The colors of A
and B will appear to match. The colors of C and D will be
different.
Scattering is also affected by the amount of air/pigment
interfaces in a paint film, as well as by the refractive index of
the pigment particles involved. At higher PVCs , there are increased
amounts of void spaces which scatter larger amounts of light that
will be further additively mixed as white. This was illustrated by
Feller and Kunz (1981) who measured the surface reflectance at 440
nm of ultramarine blue paint formulated at various PVCs (440 nm is
the wavelength at which the maximum reflectance will occur when the
paint is saturated with binder). Figure 4 shows how, for ultramarine
blue in dammar, with increasing PVC (and therefore increasing void
space which results in greater scattering at air/pigment
interfaces), the amount of reflected, diffusely scattered light
increases and the color becomes lighter.
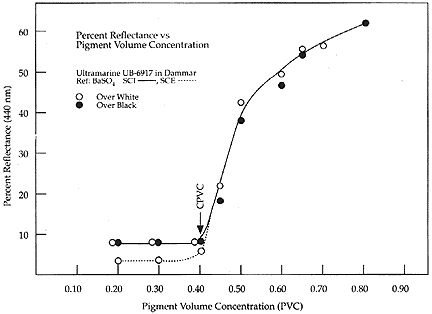
Figure 4. Graph of the percent reflectance at 400nm plotted versus
the PVC of ultramarine pigment in dammar (after Feller and Kunz
1981).
Feller and Kunz (1981) initiated their study in part to demonstrate the fact that the darkening phenomenon associated with high PVC paint was primarily a result of the filling of void spaces, and not primarily due to differences in the refractive indices of the pigment and the binder. They found a series of polymers of different refractive indices were similar in their darkening effects for ultramarine blue paint formulated at different PVCs. The conclusion that individual refractive indices of pigment and resin are relatively unimportant in the darkening that occurs when impregnating porous paint (at a very high PVC) is an important one when trying to determine the materials and methods that might be useful for consolidation treatments.
Factors affecting the deterioration of high PVC coatings are related to increased porosity and permeability and decreased strength and flexibility. Porous paint is more open to the effects of oxygen and water or humidity, and may degrade relatively faster than a less permeable coating through environmentally induced deterioration and biodeterioration. Similarly, protective effects with respect to the ground or substrate may be lessened. Other factors affecting deterioration are: the size, shape, and particle size of pigments; binder/pigment interactions; solvent systems of the vehicle; and the physics of drying, including the development of internal stress (also related to the thickness) in an applied wet coating.
Abstracts of articles on the theories of leveling of coatings and conformational coating of rough surfaces have also been included in this chapter. In the conservation literature, the most influential has been Feller's (Feller et al. 1984) discussion of control of the appearance of freshly applied varnish on paintings. Feller used a diagram similar to Figure 5 to show that, if a solvent-type varnish forms an immobile gel at a point when considerable solvent still remains, it will tend to form a surface which follows the irregularities of the paint underneath the varnish. Similarly, polymers of high viscosity grade (highly viscous at low solids content) will stop flowing over a surface sooner than resins with a low viscosity grade (less viscous at a similar solids content). Because a coating that is not able to level itself tends to form a non-glossy surface, factors that inhibit the length of time allowable for a solution to flow over a rough surface (such as a rapid loss of a highly volatile solvent) promote a matte finish.
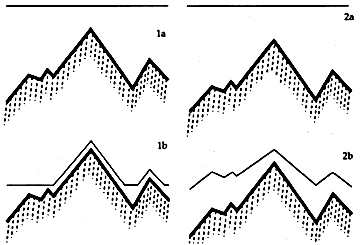
Figure 5. Leveling of solutions of high and low viscosity grade
polymers (after Feller 1985).
1a. Level of solution of low viscosity grade polymer when applied
(wet).
1b. Level of solution of low viscosity grade polymer which continues
to flow at high solids concentration while drying.
2a. Level of solution of high viscosity grade polymer when
applied.
2b. Dry film of high viscosity grade polymer which ceases to flow at
high solids concentration while drying and follows the
irregularities of the surface.
In this chapter, treatment methods and materials are discussed with reference both to the general properties of matte paint and to consolidation treatment and testing programs reported in the conservation literature. The aim is to explore as many options as possible for the treatment of matte paint. Particular emphasis is given to treatment strategies that are most likely to achieve minimum change in appearance with the required degree of consolidation. The way that this can be achieved in any given instance depends on an understanding of the relationship between the physical characteristics of the paint and the variety of treatment systems that might be suitable for use.
In order to visualize this relationship, the schematic representation shown in Figure 6 was developed. It is not intended as a guide to selecting particular materials or methods but rather as an aid to developing relevant treatment strategies. It indicates, for example, that the treatment strategy required to consolidate crumbling or flaking paint could be different from the one required to consolidate powdering paint. Even for powdering paint the strategy might vary depending on whether the paint was applied as a thick or as a thin coating. In practice, object surfaces occur in a variety of conditions ranging from flaking to crumbling or powdering within the same area; high PVC paint may be adjacent to, or layered over, paint with a high resin content; several overlaid areas of paint may each exhibit different problems, such as flaking paint on a powdering ground.
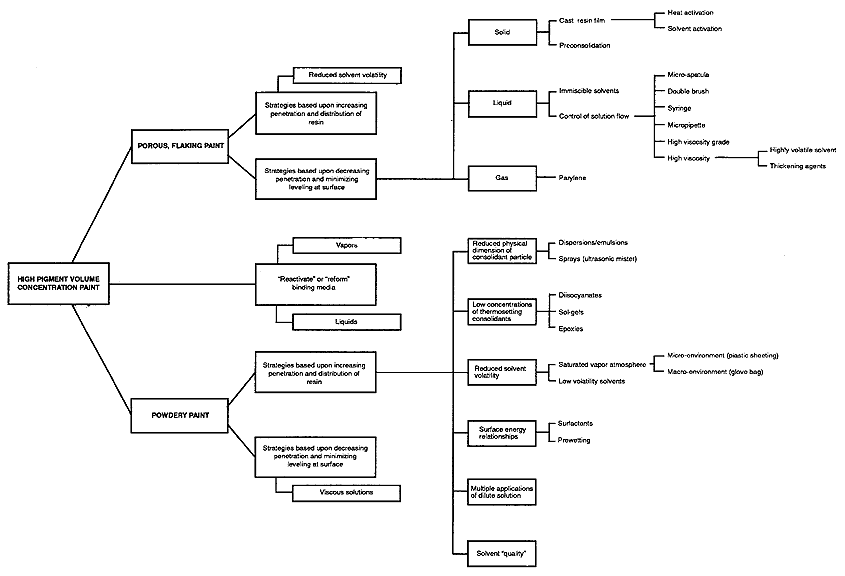
Figure 6. Schematic Representation of Paint Properties and Possible
Treatment Options
Progress in the development of satisfactory treatments for matte paint has been slow, due to the complex and conflicting nature of the problem: the need to convert highly porous, loosely bound matte paint into a coherent layer that can be safely adhered to a support without damaging the paint, or changing its color or reflectance characteristics. The tendency in the past has been to concentrate mainly on the specific properties of treatment materials to achieve the desired outcome rather than on the treatment system as a whole. This somewhat restricted approach has often been accompanied by unconfirmed or highly speculative theories to account for discoloration or the behavior of solutions, and confusing and sometimes conflicting conclusions about the stability of consolidants, their function and suitability for use on particular objects.
Discoloration is a common side effect of consolidation, usually as a result of the way that the consolidation is distributed in the paint layer. It may, however, also be due to solvent interactions or phenomena completely unrelated to appearance changes that are the result of the consolidant distribution: solubility of colorants or binders; solubility of dirt, fungus and bacteria or products of biodeterioration; and, soluble components in the substrate. Schiessl (1989) has commented on the formation of tidelines from the physical redistribution of small particles in a paint that has a wide range of particle size. The flow of a solution moves the smaller particles, but leaves the larger particles in place.
When specific conclusions about materials and methods are desired from an evaluation, explicit testing criteria must be established as a basis for quantifying effects for comparison. The variables being compared must be sufficiently isolated to allow confidence that a change in a specific variable will have a quantifiable effect. In previous comparative studies (listed in the bibliography), this may not be clear. For example, Acryloid B-72 as a 5% solids solution in toluene may be compared with a 1% aqueous gelatin solution. If the stated result is that the gelatin solution causes comparatively less change in appearance following brush application, is this due to the difference in the optical properties of the two consolidants, or to the difference in the concentrations of the two solutions, or to the difference in the polarity or volatility of the two solvents used to make the solutions? Furthermore, what differences in consolidative strength resulted from the different treatments?
For example, Feller and Kunz (1981) suggested that when very little darkening results from the addition of a consolidant, analysis of the paint will show that it is still at a high PVC and subsequently there will be little improvement in the paint cohesion. Hansen et al. (1990) reviewed practical and theoretical information and concluded that treatment parameters, specifically solution properties (such as volatility, viscosity, and surface tension), were important considerations than individual resin properties.
In addition to the difficulty in designing and carrying out comparative testing, and evaluating the results, there is the added difficulty of designing future testing when the reasons for the behavior of materials are not well understood. For example, two of the many possible explanations for the discoloration or darkening of porous powdering paint resulting from consolidation are:
Where interactions with resins are important and the PVC is comparatively low, there may be some concern for the effects of different refractive indices. As previously discussed, Feller (Hansen et al. 1990) believes that the refractive index rarely needs to be considered in high PVC systems.
Hansen et al. (1993), concurring with Domaslowski (1987-88) that the migration process is insufficiently known and understood by both scientists and conservators, used a different working assumption than "reverse migration" to account for higher resin concentrations at or near the surface of a porous paint that had been treated with a consolidant solution. Penetration of the consolidant solution into the paint was considered to be inhibited by a rise in the viscosity of the consolidant solution due to loss of solvent from evaporation, as opposed to resins first penetrating a system and then returning to the surface with solvent evaporation (reverse migration). Because suppression of solvent evaporation would diminish both a rise in viscosity as a solution flows into a system and reverse migration with solvent evaporation, the actual mechanism remains unclear.
A wide range of issues need to be addressed in addition to the chemical stability and physical properties of consolidants. The situation is even further complicated by the fact that many of the physical properties of thermoplastic polymers used in conservation, including tensile strength, extendability, and the glass transition temperature, may be dependent upon the solvent or solvent systems used to make the solution that delivers the resin (see the discussion of solvent "thermodynamic quality" below).
Walston et al. (1987) suggested that desirable properties of resins for the consolidation of crumbling and flaking paint on ethnographic objects, in addition to causing minimal change in appearance, were those that promoted: suitable working properties for the particular application techniques to be used; physical and chemical compatibility with the paint layer and support; compatibility with the range of environmental conditions that might apply after use in treatments of objects; the formation of a sufficiently flexible bond between the paint and support to ensure good adhesion between materials with different levels of expansion and contraction; and long term chemical and physical stability. In a continuation of this study, Horton-James et al. (1991) examined a wide variety of tests that could be used to evaluate the stability, appearance, and performance of resins for the conservation of porous flaking paint on ethnographic objects. They concluded that the most useful information resulted from those tests that most closely simulated the object and its environment. They also emphasized the importance of determining the aging characteristics of consolidants since it is doubtful whether they could ever be removed if, in the future, they were found to be damaging.
Articles dating from 1935 in the literature survey indicate that the range of materials used in the consolidation of fragile surfaces vary widely and that their stability and properties are equally varied. These include natural products (beeswax, dammar, gelatin, funori, etc.), synthetic polymers (poly(vinyl acetate)s, acrylics, soluble nylon, poly(vinyl butyral), epoxies, etc.), and inorganics (sol-gels). Resistance to chemical changes in the consolidant that would affect the color stability (darkening or yellowing), strength of adhesive bond, and solubility in the future are desirable qualities. References to environmentally induced chemical modifications of objects and conservation materials are not dealt with directly in this bibliography because a meaningful coverage would need a great many more entries. However, the following discussion, which is concerned only with materials that have been considered for the consolidation of paint, is included in this introductory text because the choice of materials is an important factor in treatment.
Feller (1978) suggested standards for the evaluation of thermoplastic resins for use in conservation by developing a classification scheme. The divisions were made with the following considerations: Class A materials are expected to last in excess of 100 years with less than 20% change in the properties of interest; Class B, 20 to 100 years; and Class C, fewer than 20 years (materials in this class are generally considered unsuitable for use on museum objects). The initial laboratory-based criterion was the photochemical stability of resins and polymers in relation to loss of solubility; subsequent criteria included thermal discoloration and loss of molecular weight (Feller and Wilt 1990). Materials used for the consolidation of painted ethnographic objects which may be Class A, but still remain to be fully tested and reported on in the conservation literature, were placed in Class B by Hansen et al. (1990). (This particular consideration was not used by Feller in 1978.) Although this classification scheme may be quite useful for assessing the suitability of chemical classes of resins, the problems still remain of assessing the aging characteristics of materials which vary according to manufacturer, or even from year to year by the same manufacturer, particularly in regard to emulsions which contain a wide range of additives.
A further complication is that aging characteristics vary according to treatment circumstance. Consolidants tested under controlled conditions in a laboratory may have very different characteristics when compared to those in a treatment situation. Aging properties can be affected by interaction with paint and object materials, as well as with the environment.
On the above basis, materials which may be given a Class A rating at this date are: some acrylic resins, poly(methyl methacrylate) (PMMA) and Acryloid B-72; poly(vinyl acetate) resins (in their supplied form as opposed to being a component of an emulsion or dispersion formula); and some water-soluble cellulose ethers (see Feller and Wilt 1990). Poly(n-butyl methacrylate) (PnBMA) is a component of many emulsions, but is more prone to cross-linking than PMMA or Acryloid B-72. On this basis, PnBMA-based emulsions would have a stability classification of B or C. Poly(vinyl butyral) (PVB) and ethylene vinyl acetate (EVA), also widely used, may be stable enough to be rated Class A; however, a definitive series of testing to elucidate the stability of these polymers is not known to the authors.
Poly(vinyl alcohol) (PVOH) was given a Class C rating based upon the field observations of a number of conservators and tests indicating unacceptable performance in accelerated thermal aging tests (Feller and Wilt 1990). However, it is unclear in what way PVOH has been used, i.e., whether as a resin dissolved in water or as an emulsion (a formulated commercial product). Recent testing (Bicchieri et al. 1993) has suggested that PVOH is not a particularly unstable resin. (See Feller n.d. for a more in-depth discussion of these issues).
A final comment on materials concerns the stability of gelatin. Gelatin was classified as a Class B material by Hansen et al. (1990) because, although under the right climatic conditions (low, stable relative humidity) the material may last in excess of several hundred years, it may also readily degrade if subjected to high humidities, fluctuating temperatures, and intense light levels (note the references to powdering distemper (glue-based) paint in the "History" chapter). The stability may also be influenced by the nature of the pigments (Kenjo 1974).
The use of facsimiles to help model deterioration processes or treatment systems has not been widely reported in the literature although views about their advantages and disadvantages abound. If facsimiles are to be used, it is clearly important to determine the specific properties in the paint or treated paint system that are to be simulated and to keep variables to a minimum. Simulating old and complex paint is particularly difficult when the aim is to assess the effects of proposed treatment prior to carrying out work on objects. The nature and distribution of binders within the paint layer, interaction of binder and vehicle with the substrate, pigment particle size, quantity and distribution of dirt within the paint, multiple paint layers, and the effects of variation in color and properties must all be considered.
Walston and Gatenby (1987) used facsimiles in a study aimed at selecting a treatment material for the adhesion of flaking paint on an old and very rare Australian Aboriginal painted shield. Even using the most promising materials, they found that appearance differences between treated facsimiles and a preliminary spot test on the object were very pronounced. For this reason, together with concerns about the stability of emulsions, further attempts at treating the object were abandoned. The variables of paint composition, thickness, dirt, and substrate had not been well enough reproduced in the facsimile to obtain a realistic picture of the effect that treatment materials would have on the object.
Walston and Gatenby (1987) used facsimiles in a study aimed at selecting a treatment material for the adhesion of flaking paint on an old and very rare Australian Aboriginal painted shield. Even using the most promising materials, they found that appearance differences between treated facsimiles and a preliminary spot test on the object were very pronounced. For this reason, together with concerns about the stability of emulsions, further attempts at treating the object were abandoned. The variables of paint composition, thickness, dirt, and substrate had not been well enough reproduced in the facsimile to obtain a realistic picture of the effect that treatment materials would have on the object.
Horton-James et al. (1991) made extensive use of facsimiles in tests on the properties of materials for the adhesion of flaking paint and concluded that greater use of facsimiles would have been an advantage for many of the tests, provided improvements were made in their manufacture. In this instance, there was no direct comparison between treated facsimiles and treated objects.
Facsimiles can also provide useful practice surfaces for the development or refinement of treatment techniques. This is particularly significant for porous paint where treatment materials cannot be removed and the effects of treatment need to be assessed before tests on the object, since even small spot tests can cause appearance problems.
The schema illustrated in Figure 6 is intended as a guide to help categorize paint problems and relate these problems to potentially useful treatment options. It is not intended to suggest any particular application method or material. It should be considered with the understanding that the schematicized relationships remain subject to change or reinterpretation as more data becomes available. For example, it may prove desirable to break down further the categories of paint, perhaps into groupings based upon physical properties of pigments (particle size, refractive index, etc.) or the chemical composition of the binders.
The different treatment methods in the schema have been categorized in relation to the problems characteristic of the paints (flaking and porous, or powdering) and in relation to delivering a consolidant in a desired distribution (internally or at the surface). Because consolidants are usually delivered by brush or spray application of a solution, the factors affecting solution flow are of primary interest, particularly viscosity.
The simplest definition of viscosity, found in most dictionaries, is "the resistance of a solution to flow". Of greatest importance to this discussion is the dependence of viscosity on the amount of polymer dissolved in the solvent; as the concentration increases, the viscosity becomes greater. When a polymer solution reaches a certain concentration through solvent evaporation it will essentially cease to flow. It is at this point that the distribution of the consolidant becomes fixed.
Therefore, highly volatile solvents and high-viscosity-grade resins can be used to form concentrated solutions that can be put to use either to promote the retention of surface roughness by inhibiting leveling or by decreasing penetration of the consolidant solution and the filling of void spaces by the addition of a consolidant. Conversely, by using an initially very low concentration solution, a low volatility solvent, or a means of slowing or inhibiting solvent evaporation, the flow and spreading or penetration of consolidants will be promoted.
A model of how inhibition of solvent evaporation may reduce darkening and the formation of tidelines outlining the extent to which a consolidant solution has flowed through a paint layer is shown in Figures 7, 8, and 9. Figure 7 shows the flow of a solution (in cross section) while solvent is simultaneously evaporating from the surface. The concentration (and therefore the viscosity) is increasing with time due to solvent evaporation as the solution flows both into the porous paint and spreads outward. This results in higher concentrations of consolidant at the surface and at the front of solution flow, causing increased gloss and darkening, depicted in Figure 8. Figure 9 shows the contrasting situation where solvent loss is suppressed by maintaining a vapor-saturated atmosphere. The solution flows into and throughout the porous paint layer with little final differentiation in the concentration of resin.

Figure 7. Schematic of resin solution applied to a porous paint
surface in an open atmosphere (after Hansen et al. 1993).
align="center" Figure 7.
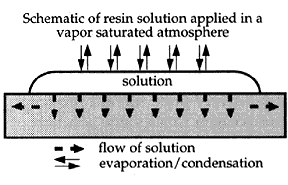
Figure 8. Schematic of resin solution applied to a porous paint
surface in a vapor-saturated atmosphere (after Hansen et al. 1993)
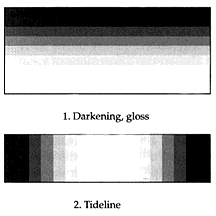
Figure 9. Possible concentration profiles of resin in a consolidated
paint layer, shown in cross section, which result from application
in an open atmosphere which allows solvent evaporation (darker areas
indicating higher concentrations of resin (after Hansen et al.
1993).
Referring again to the schema shown in Figure 6, porous paint is categorized into two subcategories: porous and powdering; and porous and flaking. Although strategies for promoting or inhibiting solution flow can be applicable to both categories, in general those that promote the penetration and distribution of the consolidant are most often considered in relation to powdering paint; and those that inhibit penetration or minimize leveling of a surface coating are considered in relation to porous, flaking paint.
It has often been observed by conservators that exposure to solvents or solvent vapors alone seems to "reform" and possibly "reactivate" the binder already present in either flaking or powdering paint (e.g., when an object is placed in a humid atmosphere for some time to relaxflaking paint prior to readhering the flakes, this seems to promote adherence of the flakes). It is important to include this phenomenon because many field observations to this effect made by conservators are known to the authors. However, because there are also no known systematic studies relating types of binders, pigments, and solvents to this phenomenon in porous paint, the ramifications and veracity of the "reforming" of resins remains open and subject to debate.
Referring to Figure 6, it can be seen that the widest variety of methods that may be useful for the consolidation of powdering paint (reported in the literature to date) are related to the penetration of an applied solution and the final distribution of the consolidant throughout the paint layer. Three of these are based upon the control of solvent volatility: 1) solutions prepared with low volatility solvents (such as diethyl benzene) described by Welsh (1980); 2) evaporation inhibition through saturating the working environment with solvent vapors (Hansen et al. 1993); and 3) multiple applications of dilute solutions which penetrate to a greater depth due to their initial low viscosity.
Increased penetration may also occur if the particle size of emulsions is reduced, allowing them to flow into small pores. Koob (1981) has suggested that using those dispersions of polymers that have an effectively smaller size than an emulsion of the same material will promote this penetration.
Michalski (Anon. 1990) has suggested using an ultrasonic humidifier to produce a spray of a solution with a very small droplet size. This spray could penetrate to a greater extent and deliver a smaller amount of solution to a surface in a controlled manner, thus reducing the concentration of the consolidant delivered to the paint in each individual application.
Another technique employed to minimize the concentration of resin in both the vehicle and the solid state is the use of thermosetting polymers which polymerize or set in situ. These materials, although not soluble, form a strong bond if they react chemically with the pigment particles orsubstrate. In general, greater consolidative strength would result with a lesser amount of material in comparison to soluble but non-reactive thermoplastic resins which mechanically adhere pigment particles together (Hansen and Agnew 1990). Both organic materials (such as epoxies, Weintraub and Greenland 1984) and inorganic materials (such as sol-gels, Romich et al. 1990) in low concentrations have been suggested for fixing powdering paint.
Another factor which reduces penetration is improper wetting (see Patton 1979 for a discussion of surface energy). Two methods have been suggested to improve wetting:
The use of solvents of different thermodynamic "quality" has also been investigated to control the distribution of a resin within a porous system. "Good" solvents for a particular polymer can dissolve larger amounts of a material in comparison to "poor" solvents. Thus, polymers precipitate out at a lower concentrations in poor solvents than good solvents, so that with solvent evaporation they also precipitate sooner. Gerassimova et al. (1975) found that impregnation of loose plaster with a 10% PBMA solution in xylene-ethyl alcohol (a comparatively poor solvent mixture) in comparison to a xylene solution of the same concentration (a comparatively better solvent) gives more regular resin distribution inside the porous material and considerably less change in appearance. This agrees with Domaslowki's findings (1987-88) that poorer solvents promoted less surface concentrations of acrylic resins in porous stones, although the effect of resin migration depended not only on the quality of the solvent but also on the dimensions of resin molecules, solution viscosity, stone structure, and the conditions of drying after impregnation. Gerassimova and Melnikova (1978) subsequently found that, although the resin was distributed more evenly, the physical properties (porosity, water vapor absorption, bending strength) were unaffected by using solvents of different quality to deliver the PBMA.
Because possibilities for improving compatibility with a substrate, such as manipulating flexibility and strength, have been demonstrated in relation to solvent quality for some polymers used in conservation (Hansen et al. 1991, Hansen 1994 in press), further investigation of these phenomena in a system such as porous paint may result in even more available techniques for consolidation. For example, tensile testing of solution cast films (subsequent to nearly full solvent release) has shown that films of Acryloid B-72 cast from acetone solutions have low extendability (under 10% strain-to-break) in contrast to films cast from toluene solutions (over 100% strain-to-break). Thus, application of Acryloid B-72 from a toluene solution may be more suitable than an acetone solution if the paint being consolidated is on a flexible substrate such as a textile.
Strategies based upon minimizing leveling (suggested by a number of field observations made by conservators known to the authors) include multiple spraying of the surfaces of pastels with extremely viscous solutions in high volatility solvents. Presumably, there is little penetration but enough resin is delivered to the surface to hold particles together without forming a smooth film.Spray application of a viscous polymer solution made with a highly volatile solvent has been successfully used for flaking paint, and may be particularly applicable to large areas of flaking paint on objects (Mibach 1990). However, there is still some concern that the adhesion to the substrate may be unaffected or negatively affected by the fixing of a paint film only on the surface. Because there is yet to be published information relating solvents, resins, pigments, paint thickness, and adhesion to the substrate, little comment can be made other than to point out the necessity for confirmative testing of spray techniques for the consolidation of powdering paint.
The second category is paint characterized by the lifting of porous flakes. Because adhesive solutions applied under a porous flake may wick into the flake causing discoloration, methods that discourage penetration of the consolidant have been considered, along with consolidants that conformally coat a flake and retain the matte appearance.
Reactivation of a solid (cast resin film) placed under a flake by heat or wetting with a solvent tomake the film tacky, but not enough to induce flow, is often used. Hatchfield (1988) reported some success in maintaining the appearance of water-soluble paints by using a preconsolidant of a cellulose ether in ethanol to strengthen flaking paint and then adhering the flakes with an aqueous solution of methyl cellulose.
Common techniques focusing on the control of solution flow or the application of minimal amounts of solution that can only flow to a limited extent are:
Another technique for paints that interact negatively with either water or organic solvents, is saturation of the porous flakes with a solvent immiscible with the solvent used to make the consolidant solution. For example, water-sensitive flaking paint has been successfully adhered with acrylic emulsions while the paint was saturated with toluene (which did not allow wicking of the emulsions into the paint layer), and then the toluene was allowed to dry, leaving the flaking paints still porous but adhered (Futernick 1990, personal communication).
Methods that promote penetration may have some use in adhering porous flakes, particularly if enough resin is delivered to the interface of the flake and substrate to adhere the flake. Application of a consolidant solution in a vapor-saturated atmosphere has been used with some success on ethnographic objects with extensive flaking over a large area. However, Hansen and Volent (1994 in press) have pointed out some practical limitations to this procedure in a cautionary note on solvent sensitivity testing.
A novel method is the application of a conformal coating, Parylene, (Humprey 1984; Grattan 1989) that polymerizes from the gaseous state onto the surface of a paint layer, thus in theory conforming almost completely to the surface roughness. The practical benefits and limitations of using this material in this manner have yet to be fully demonstrated.
Lastly, another method mentioned by many conservators is the use of matting agents. Silica or other inert, low-refractive-index "fillers" are mixed with the consolidant to reduce gloss. However, matting agents tend to lighten or alter the color of the treated surface compared to the untreated surface, thus making it difficult to match the original color (especially if spot treatments are required).
The subject of retreatment of unsuccessful treatments deserves some additional comment. As stated above, because the reasons for changes in appearance may be related to the distribution of a consolidant in a porous paint system, these changes can be minimized by further treatment based upon an understanding of the same principles. For example, localized surface concentrations of consolidants may be reduced and the consolidant redistributed (by using several techniques mentioned in the schema); however, this would only be possible when soluble consolidants were originally used.
The sections of Matte Paint printed here represent 17 of the 61 pages of the Introduction; not included were "History and Technology" and "Analysis". For the numerous references cited in the article, readers should consult the original publication.
Matte Paint
Its history and technology, analysis, properties, and treatment,
with special emphasis on ethnographic objects
Eric F. Hansen, Sue Walston, and Mitchell Hearns Bishop, Editors
Published by the Getty Conservation Institute, Autumn 1994
ISBN: 0-89236-262-6
Price: $45
* Throughout the bibliography and this introductory "topical review", reference is made to literature that states certain polymers were used, or certain methods were found useful, to consolidate paint on objects. However, these treatments often have not been tested or monitored over time. The authors wish to emphasize that they are reporting, and in some instances commenting on, what has been published; they are not assessing the suitability of a procedure nor the "success" of a treatment for specific objects.