EVALUATION OF APPEARANCE AND FADING OF DAYLIGHT FLUORESCENT WATERCOLORSSANDRA A. CONNORS-ROWE, HANNAH R. MORRIS, & PAUL M. WHITMORE
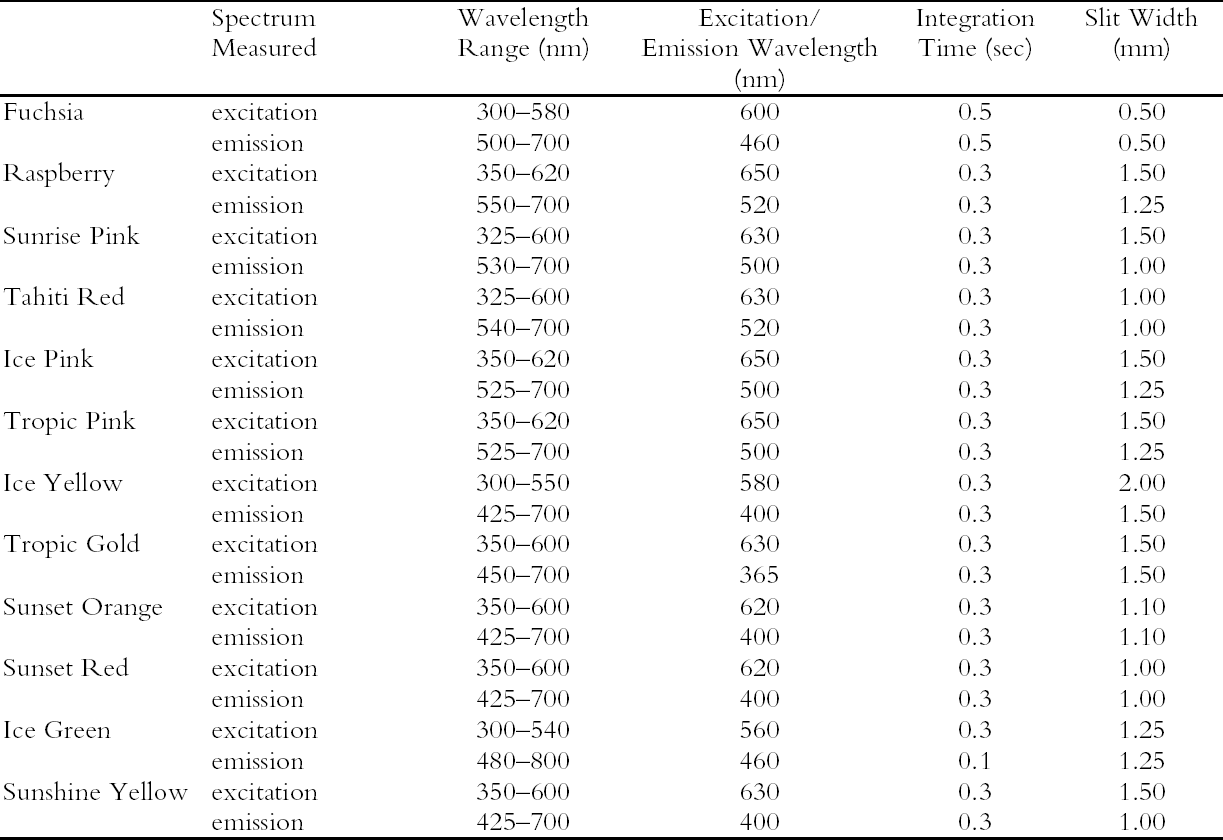
NOTES1. Two lamp types can be used as black light sources. Both are UVA-emitting lamps with peak intensity at 365 nm in the UV and narrow energy peaks at 440 nm and 550 nm in the visible region. One lamp type retains the visible light intensities, and the other filters the intensities above 400 nm using a dark blue filter. The latter of these two lamps was used for the black light experiments in this study, so the behavior of these watercolors under UV wavelengths alone could be explored. 2. The change in fading rate was calculated from the slope of the Δ E curves for both UV-included and UV-excluded simulated daylight exposure between 0 and 1.3 Mlux-hours. The ratio of the slope of the UV-excluded curve to the slope of the UV-included curve was multiplied by 100 to give the percent of unfiltered fading rate. APPENDIXAPPENDIX: EXPERIMENTALThe 12 fluorescent watercolors (Fuchsia, Raspberry, Sunrise Pink, Tahiti Red, Ice Pink, Tropic Pink, Ice Yellow, Tropic Gold, Sunset Orange, Sunset Red, Ice Green, and Sunshine Yellow) from the Dr. Ph. Martin Radiant Concentrated Water Color line of products were studied. Samples were prepared by applying each watercolor, by brush, to Arches hot press watercolor paper and then allowing them to dry. Seven replicate samples were prepared for exposure to various lighting conditions (described below). Multiple applications of the watercolor were made when necessary to ensure that replicates had equivalent initial depths of shade. Samples with a range of color concentrations (low to high) were prepared by application of diluted watercolor paint to paper. Pairs of replicate samples were exposed to each of the following accelerated aging conditions: (1) xenon lamp exposure with UV included, (2) xenon lamp exposure with UV excluded, and (3) black light (F20T12/BLB from General Electric). UV-absorbing Plexiglas (UF3) sheets were used to filter out the UV from the xenon lamp source when necessary. A Heraeus Suntest CPS, which employs a xenon source filtered to eliminate infrared and short wavelength ultraviolet, was used for light exposure. Samples were placed on a watercooled sample tray held at 20�C with recirculating chilled water. The intensity of light in the Suntest CPS, measured by a radiometer in lux for a diffuse light source (International Light IL1700 with detector SED010 351, filter Y 70, and diffuser W 323), was 5.45 � 104lux with UV included in the output and 4.96 � 104lux with UV excluded from the output. The intensity under black lights, measured by a radiometer for total ultraviolet radiation (International Light IL1700 with detector SED400 383, filter 20162, and diffuser W315) was 1.63 � 10-3 W/cm2. Aging experiments were also conducted under either high-output daylight fluorescent lamps (General Electric F48T12-D-HO) or facing a north window, with similar results obtained. Following exposure under accelerated light-aging conditions, some reversion of the faded fluorescent color was observed if the samples were allowed some period of dark storage prior to color measurement. For consistency, color measurements were made immediately after samples were removed from their exposure conditions. These data were compared to naturally aged samples (daylight exposure through a north-facing window). The data from artificially aged samples were consistent with the natural aging result. However, only the results of the artificial aging tests are reported. Color measurements, for samples exposed to simulated daylight conditions, were made using a GretagMacbeth Color Eye 7000 Colorimeter. The reflectance data collected by this instrument (specular reflectance excluded) include both the directly reflected light and the emitted fluorescence. A UV filter was placed in the optical path before the light reached the sample to measure appearance changes both with and without UV wavelengths. Optiview version 1.5 software for this instrument provided reflectance data as well as tristimulus values calculated for 2� observer and standard illuminant D65. Color differences were calculated with the 1976 CIE L*a*b* formula. The spectra of the fluorescent emission under black light illumination were measured directly, without reference to a white standard, using a fiberoptic-based photodiode array spectrophotometer (Control Development Model PDA-512-USB). Under these conditions, the emission spectra of some of the watercolor samples (Raspberry, Tahiti Red, Tropic Pink, Ice Pink, Fuchsia, and Sunrise Pink) were so weak that the spectra displayed artifacts from the black light and the optical brighteners in the paper support. These artifacts were removed from the spectra by setting the intensities in the 400–515 nm region to the value Reflectance measurements used to determine appearance under light sources with different corre-lated-color-temperatures (CCT) were also made with the fiberoptic-based spectrophotometer, using a yellow filter (Schott Glass Technologies FG-13) to lower the CCT of the xenon lamp used as the illumination source. The measured intensities were normalized to 1.0 at 700 nm (a wavelength that is not likely to be affected by the change in CCT) after collection. Fluorescence excitation and emission spectra were collected using a Fluorolog ISA (Jobin Yvon, Horiba Group). Samples on watercolor paper were cut into 4 cm � 13 mm strips and placed inside a quartz fluorescence cuvette for analysis. The paper samples were positioned so that the exciting light beam was directed at about a 45δ angle to the paper surface, and the emitted fluorescence was collected at about a 45� angle. A complete list of experimental conditions can be found in table 5. ACKNOWLEDGEMENTSThis work was performed at the Art Conservation Research Center at Carnegie Mellon University and was supported by a grant from the Andrew W. Mellon Foundation. The authors would like to thank Dr. Susan Daly and Dr. Robert Tilton of the Biomedical Engineering Department at Carnegie Mellon University for the use of their Fluorolog ISA fluorimeter.
REFERENCESBaer, N. S., A.Joel, R. L.Feller, and N.Indictor. 1986. Indian yellow. In Artists' pigments: A handbook of their history and characteristics. Vol. 1, ed. R. L.Feller. Washington, D. C.: National Gallery of Art. 17–36. Baxter, E.2003. Personal communication. Carnegie Museum of Art, Pittsburgh, Pa. Berns, R. S.2000. Billmeyer and Saltzman's principles of color technology. 3rd ed. New York: John Wiley and Sons. Billmeyer, F. W., and L. B.Hepfinger. 1983. Energy transfer between fluorescent organic pigments. Color Research and Application8(1):12–16. Christie, R. M.1993. Fluorescent dyes. Review of Progress in Coloration23:1–18. Dane, C.1977. Fluorescent colourants and their use in printing inks. British Ink Maker20(1):11–13. de la Rie, R.1982a. Fluorescence of paint and varnish layers. Part 1. Studies in Conservation27:1–7. de la Rie, R.1982b. Fluorescence of paint and varnish layers. Part 2. Studies in Conservation27:65–69. Ellis, M. H., C. W.McGlinchey, and E.Chao. 2002. Daylight fluorescent colors as artistic media. In The broad spectrum: Studies in the materials, techniques, and conservation of color on paper, ed. H. K.Stratis and B.Salvesen. London: Archetype Publications. 160–66. Johnston-Feller, R.2001. Color science in the examination of museum objects: Nondestructive procedures. Los Angeles: Getty Conservation Institute. Johnston-Feller, R., and C.Bailie. 1982. An analysis of the optics of paint glazes: Fading. In Science and technology in the service of conservation, ed. N. S.Brommelle and C.Thomson. London: IIC. 180–85. Meadows, W.1974. Formulating with fluorescent pigments. Paint and Varnish Production64(9):31–37. Smith, T.1982. Luminescent and fluorescent pigments for printing inks. Polymers Paint and Colour Journal172(4077):542, 545–46. Tsang, J., S. E.Pinchin, K.Almond, and C. S.Tumosa. 2004. Conservation of murals in the Alameda theatre: Reviving former cutting-edge fluorescent paint and black-light technology. In Modern art, new museums, ed. A.Roy and P.Smith. London: IIC. 185–88. Voedisch, R. W.1973. Luminescent pigments, organic. In Pigment handbook, Vol. 1, Properties and economics, ed T. C.Patton. New York: John Wiley and Sons. 891–903. Whitmore, P. M., and C.Bailie. 1997. Further studies on transparent glaze fading: Chemical and appearance kinetics. Journal of the American Institute for Conservation36:207–30. FURTHER READINGLakowizc, J. R.1999. Principles in fluorescence spectroscopy. 2nd ed. New York: Kluwer Academic/Plenum. Lower, E. S.1996. Fluorescent paints and pigments. Pigment and Resin Technology25(3):15–18. Yoshizawa, A.2000. Daylight fluorescent pigments in works of art: Properties, history and fading. Master of Art Conservation thesis, Queen's University, Kingston, Ontario. SOURCES OF MATERIALSDr. Ph. Martin Radiant Concentrated WaterColors Dick Blick Art Materials P.O. Box 1267 Galesburg, Ill. 61402-1267 (800) 828-4548 www.dickblick.com Arches hot press watercolor paper, 160 lb.Utrecht Art Supplies 1930 East Carson St. Pittsburgh, Pa. 15203 (412) 432-1945 Colorimeter (Color Eye 7000)GretagMacbeth 617 Little Britain Rd. New Windsor, N.Y. 12553 Jobin Yvon Inc. Horiba Group 3880 Park Ave. Edison, N.J. 08820 (732) 494-8660 www.jyhoriba.com Photodiode array spectrophotometer (ModelPDA-512-USB) Control Development, Inc. 2633 Foundation Dr. South Bend, Ind. 46628 (574) 288-7338 www.controldevelopment.com Light exposure apparatus (Heraeus Suntest CPS)Atlas Electric Devices Co. 4114 North Ravenswood Ave. Chicago, Ill. 60613 (773) 327-4520 Black lights (General Electric modelF20T12/BLB) Grainger, Inc. 3150 Liberty Ave. Pittsburgh, Pa. 15201-1416 (412) 281-4477 www.grainger.com 75W xenon arc lamp (cat. no. 6251)Spectra-Physics, Inc. 150 Long Beach Blvd. Stratford, Conn. 06615 (203) 377-8282 www.spectra-physics.com Blue Wool reference cardsTalas 20 West 20th St., 5th Floor New York, N.Y. 10011 (212) 219-0770 www.talasonline.com Radiometer (model IL1700)International Light, Inc. 17 Graf Rd. Newburyport, Mass. 01950-4092 (978) 465-5923 www.intl-light.com Low CCT filter (FG-13)Schott Glass Technologies, Inc. 400 York Ave. Duryea, Pa. 18642 (570) 457-7484 www.us.schott.com AUTHOR INFORMATIONSANDRA A. CONNORS-ROWE received a BS in chemistry from Wayne State University. During this time she gained experience in collections management at the Henry Ford Museum in Dearborn, Michigan, and as a painting conservation technician at Conservation and Museum Services in Detroit. Following her BS degree, Connors-Rowe received a master of art conservation degree with a concentration in conservation science from Queen's University in Kingston, Ontario. Since 1998, she has been a conservation scientist with the Art Conservation Research Center at Carnegie Mellon University. Her research has been directed toward noninvasive methods for evaluating the degradation of materials. Address: Carnegie Mellon University, 700 Technology Dr., Pittsburgh, Pa. 15219. E-mail: scon-nors@andrew.cmu.edu HANNAH R. MORRIS received a PhD in analytical chemistry from the University of Pittsburgh, where her research focused on materials characterization in complex polymer blends using spectroscopy and chemical imaging techniques. Since 2000 she has been deputy director of the Art Conservation Research Center. Address as for Connors-Rowe PAUL M. WHITMORE received a PhD in physical chemistry from the University of California at Berkeley. Following an appointment at the Environmental Quality Laboratory at Caltech studying the effects of photochemical smog on works of art, he joined the staff of the Center for Conservation and Technical Studies at Harvard University Art Museums. Since 1988 he has been director of the Art Conservation Research Center at Carnegie Mellon University, where his research has been directed toward the study of the permanence of modern art and library materials. Address as for Connors-Rowe |