EVALUATION OF APPEARANCE AND FADING OF DAYLIGHT FLUORESCENT WATERCOLORSSANDRA A. CONNORS-ROWE, HANNAH R. MORRIS, & PAUL M. WHITMORE
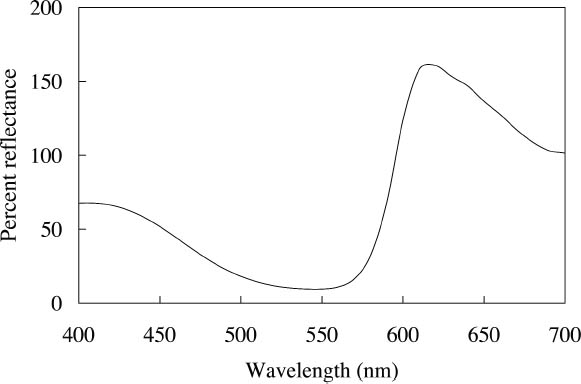
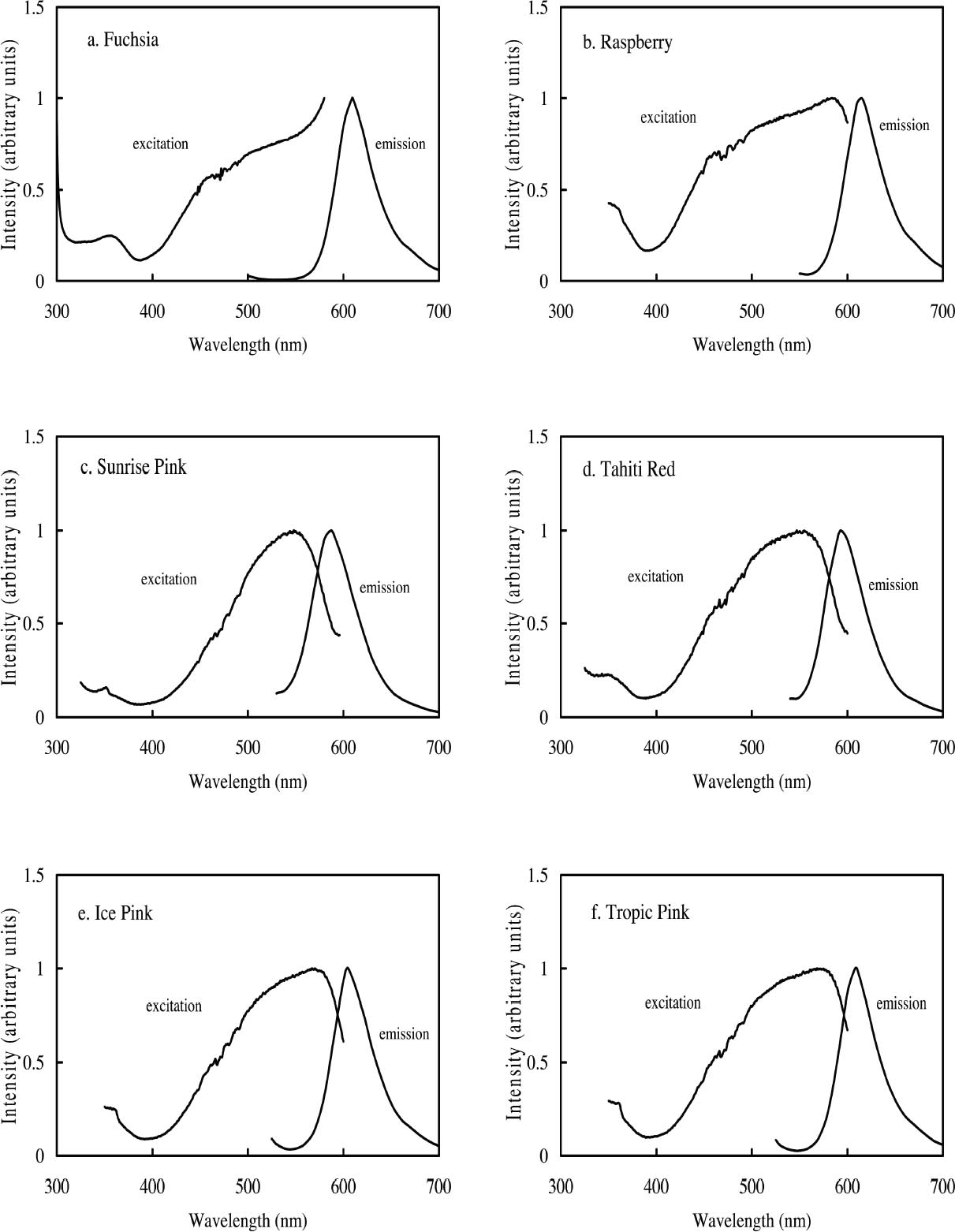
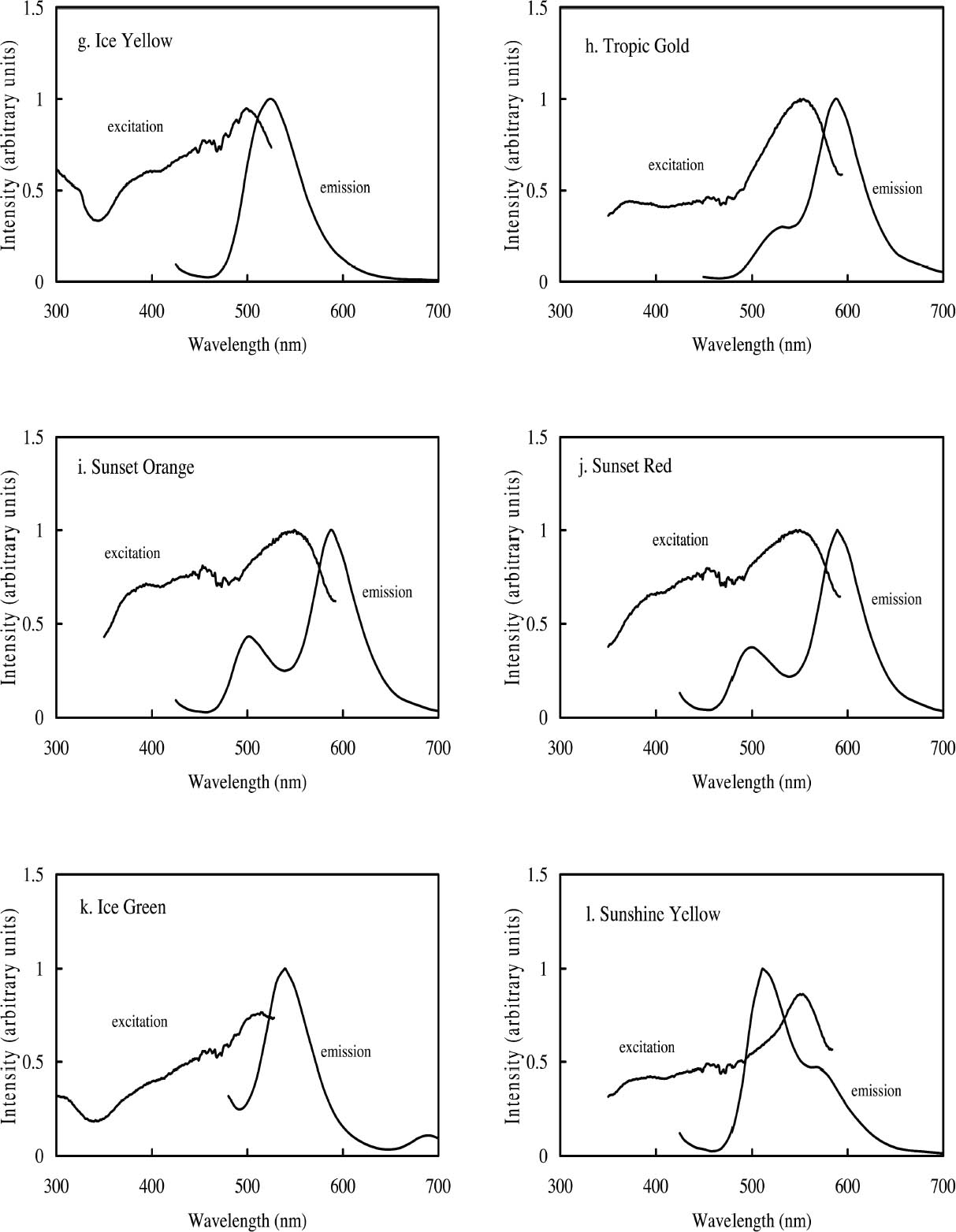
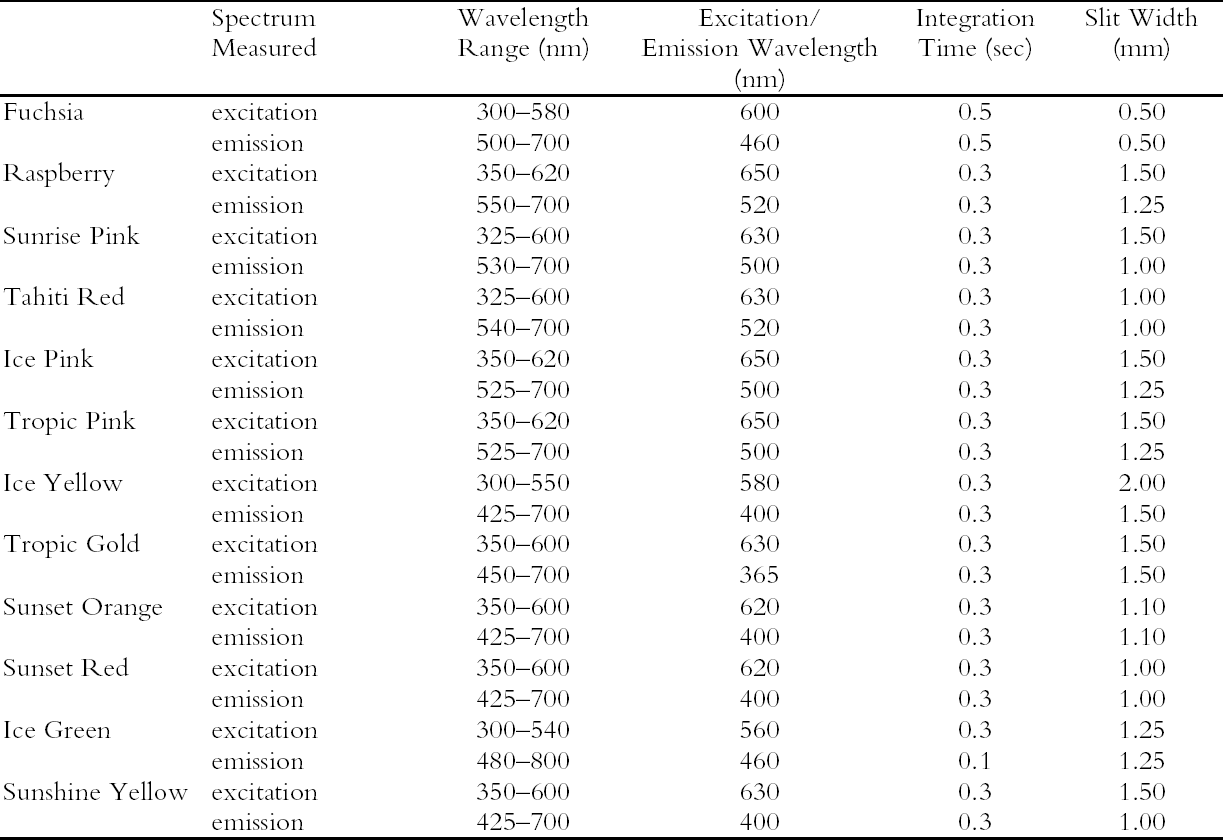
ABSTRACT—Twelve daylight fluorescent watercolors from the Dr. Ph. Martin Radiant Concentrated Water Color line of products were studied to evaluate their appearance and light sensitivity under various lighting conditions—high and low correlated-color-temperature sources, simulated daylight sources with ultraviolet light both included and excluded, and black light. The appearance tests showed that all watercolors experienced a significant reduction in fluorescent emission when a low correlated-color-temperature light source was used compared to a high correlated-color-temperature source. Only a slight change in appearance was experienced by five of the watercolors studied when UV wavelengths were excluded from a simulated daylight source, while others experienced no change at all. Nine of the watercolors showed a significant reduction in fading rate during prolonged exposure to simulated daylight from which the UV content was excluded. Black light exposure produced fading patterns similar to those found with simulated daylight exposure. However, some of the watercolors were relatively stable when exposed to black light compared with their fading rates when exposed to simulated daylight. The conservation issues related to fluorescent materials, including difficulties with matching color and inferring original appearance, were also examined. Six of the 12 watercolors showed promising results for the possibility of using dilutions of the original fluorescent material to match faded versions of that watercolor. TITRE—Une �valuation de l'apparence et de la d�coloration des aquarelles fluorescentes � la lumi�re du jour. R�SUM�—On a �tudi� douze aquarelles fluorescentes � la lumi�re du jour, de la marque Dr. Ph. Martin Radiant Concentrated Water Color (aquarelles radiantes concentr�es du Dr. Ph. Martin) dans le but d'en �valuer l'apparence et la sensibilit� � la lumi�re sous diverses conditions d'�clairage, dont des sources de lumi�re similaires � haute et basse temp�ratures de couleur, des sources artificielles de lumi�re du jour avec et sans ultraviolets, et des sources de lumi�re ultraviolette. Les tests sur l'apparence ont d�montr� qu'il se produit une r�duction marqu�e de l'�mission fluorescente de toutes ces aquarelles lorsqu'une source lumineuse de temp�rature de couleur basse est utilis�e compar� � une source de temp�rature de couleur �lev�e. On a not� un l�ger changement d'apparence chez seulement cinq des aquarelles �tudi�es lorsque les ondes ultraviolettes �taient retir�es d'une source artificielle de lumi�re du jour, alors que les autres aquarelles n'ont pr�sent� aucun changement. Neuf des aquarelles ont pr�sent� une baisse marqu�e du niveau de d�coloration lors d'une exposition prolong�e � une lumi�re du jour artificielle sans ultraviolets. Une exposition � la lumi�re ultraviolette a produit des sch�mas de d�coloration similaires � ceux obtenus lors d'une exposition � la lumi�re du jour artificielle. Cependant, certaines des aquarelles expos�es � la lumi�re ultraviolette �taient relativement stables si l'on compare leur niveau de d�coloration � celui obtenu en lumi�re du jour artificielle. Lors de cette �tude, on s'est aussi pench� sur les enjeux que posent les mat�riaux fluorescents pour la conservation et la restauration, dont les difficult�s � reproduire et � d�terminer l'apparence originale des couleurs. Six des douze aquarelles ont pr�sent� des r�sultats prometteurs quant � la possibilit� de les diluer pour reproduire la couleur des versions d�color�es. TITULO—Evaluacion de la apariencia y la decoloraci�n de acuarelas fluorescentes en luz de d�a. RESUMEN—Se estudiaron doce acuarelas fluorescentes en luz de d�a provenientes de la l�nea de productos Dr. Ph. Martin Radiant Concentrated Water Color (Acuarelas Concentradas Radiantes del Dr. Ph. Martin) para evaluar su apariencia y su sensibilidad a la luz bajo diversas condiciones de iluminaci�n — fuentes de correlaci�n color-temperatura alta y baja-, se simularon fuentes de luz de d�a con luz ultravioleta incluida y no incluida, y con luz negra. Las pruebas de apariencia demostraron que todas las acuarelas experimentaban una reducci�n significativa en la emisi�n fluorescente cuando se usaba una fuente de luz de correlaci�n color-temperatura baja, comparada con una fuente de correlaci�n color-temperatura alta. En cinco de las acuarelas estudiadas se apreci� s�lo un ligero cambio en la apariencia cuando se exclu�an las ondas de magnitud UV en la luz de d�a simulada, mientras que otras no reflejaban ning�n cambio. Nueve de las acuarelas mostraron una reducci�n significativa en la tasa de decoloraci�n durante la exposici�n prolongada a la luz de d�a simulada de la cual el contenido UV estaba excluido. La exposici�n a luz negra produjo series de decol-oraci�n similares a las encontradas con la exposici�n a la luz de d�a simulada. Sin embargo, algunas de las acuarelas T�TULO—Avalia��o da Apar�ncia e Descolora��o de Aquarelas Luz-Florescente. RESUMO—Doze aquarelas luz-florescentes da linha de produtos Dr. Ph. Martin Radiant Concentrated Water Color (Dr. Ph. Martin Aquarelas Radiantes Concentradas) foram estudadas para avalia��o de sua apar�ncia e sensibilidades � luz sob v�rias condi��es de ilumina��o — altas e baixas fontes correlatas de temperatura de cor, e luz negra. Os testes de apar�ncia mostraram que todas as aquarelas apresentaram redu��o significativa de emiss�o fluorescente quando uma fonte baixa de luz de temperatura de cor correlata foi utilizada em compara��o ‘a uma fonte alta de luz de temperatura de cor correlata. Observou-se somente uma pequen�ssima mudan�a na apar�ncia de cinco aquarelas estudadas, quando as ondas UV foram exclu�das da fonte simulada de luz natural, enquanto outras n�o apresentaram nehuma mudan�a. Nove aquarelas apresentaram redu��o significativa do n�vel de descolora��o durante exposi��o prolongada � luz natural simulada, da qual os raios UV foram exclu�dos. Exposi��o � luz negra produziu padr�es de descolora��o similares aos encontrados durante � exposi��o � luz natural simulada. Todavia, algumas das aquarelas mativeram-se relativamente est�veis quando expostas � luz negra, comparando-se seus n�veis de descolora��o quando expostas � luz natural simulada. Foram tamb�m examinados os aspectos de conserva��o relacionados a materiais fluorescentes, incluindo dificuldades no ajuste de cores e interfer�ncia na apar�ncia original. Seis das doze aquarelas mostraram resultados promissores para o poss�vel uso de dilui��es do material fluorescente original para ajustar descolora��es das aquarelas. 1 INTRODUCTIONThe introduction of fluorescent colorants constitutes a significant advance in 20th-century color technology. Originally developed for use in applications where high visibility was required, such as coloration of road construction markers or search and rescue vehicles, they were eventually used to develop a variety of fluorescent artist's materials. With these new materials came a new set of conservation concerns. Fluorescent colorants have been observed to degrade rapidly when exposed to light, particularly during prolonged exposure (Voedisch 1973). Preservation measures typically employed when exhibiting fugitive materials—restricting the wavelength region of the illuminating light source and decreasing the intensity of illumination—may impact fluorescent colorants by changing their luminous appearance, dependent as it is on both reflected light and fluorescent emission. Thus appearance changes that occur in fluorescent colorants can be difficult to anticipate or to match during conservation treatments such as inpainting. The goal of this study is to provide a better understanding of fluorescent materials and their fading behavior so that museum professionals may be better able to make exhibition and conservation decisions. Currently, a wide variety of fluorescent art materials is available, including pigments, dyes, inks, and watercolors. However, only a small number of fluorescent dyes (most commonly, CI Basic Violet 10, CI Basic Red 1, CI Acid Yellow 73, CI Solvent Yellow 44, CI Acid Yellow 7, CI Disperse Yellow 232, CI Basic Yellow 40, and CI Disperse Yellow 11) are used to create these art materials (Smith 1982; Christie 1993). In this study, 12 fluorescent watercolors from the Dr. Ph. Martin Radiant Concentrated Water Color line of products (Fuchsia, Raspberry, Sunrise Pink, Tahiti Red, Ice Pink, Tropic Pink, Ice Yellow, Tropic Gold, Sunset Orange, Sunset Red, Ice Green, and Sunshine Yellow) were chosen as examples of fluorescent materials used by both commercial and fine artists and likely to contain the typical fluorescent dyes mentioned above. The appearance changes of these watercolors under various lighting conditions (high and low correlated-color-temperature [CCT] sources, simulated daylight sources with ultraviolet wavelengths both included and excluded, and a black light source)1 and from prolonged exposure to these lighting conditions were explored along with the preservation benefits gained from the exclusion of UV wavelengths from a simulated daylight source. Consideration is also given to the conservation issues that surround fluorescent materials, specifically the attempt to match the fluorescent color on an artwork during treatment and to infer the original appearance. 2 FLUORESCENT COLORANTS AND THEIR APPEARANCE2.1 FLUORESCENT APPEARANCEAs the name indicates, fluorescent colorants derive their unique appearance from their ability to strongly fluoresce, that is, to absorb light at one wavelength and to re-emit it at longer wavelengths. The appearance of a fluorescent colorant is thus the additive mixture of directly reflected light and the fluorescent emission from other absorbed wavelengths. A reflectance spectrum of a typical fluorescent colorant is shown in figure 1 and displays the wavelength regions of low reflectance as well as the very high measured intensity due to the fluorescent emission. The addition of fluorescent emission causes the colorant's measured reflectance to be greater than the 100% limit of totally reflected incident light, and this enhanced reflectance creates the characteristic luminous appearance of a fluorescent color. In many cases the added fluorescent emission occurs at the edge of the colorant's absorption band, a situation that increases the steepness and height of the transition from low to high reflectance. This condition effectively increases the chroma of the color. These attributes—the apparent luminosity and the high chroma—are the distinctive features of fluorescent colors. The fluorescent watercolors examined in this study are examples of so-called daylight fluorescent colorants. Unlike familiar art materials such as madder lake (de la Rie 1982a), Indian yellow (Baer et. al 1986), or damar (de la Rie 1982b), which fluoresce only when illuminated with ultraviolet wave-lengths, daylight fluorescent colorants have been developed to fluoresce from exposure to visible light. Shown in figure 2 are the excitation spectra (i.e., the spectrum of light that will excite the fluorescent emission) for the fluorescent watercolors, applied on paper, included in this study. Also shown in this figure are the spectra of the fluorescent emission for these watercolors. Both excitation and emission spectra were measured using a fluorimeter (see table 5 for experimental details). All the watercolors show fluorescent excitation in the visible region between 400 and 600 nm, and many show additional fluorescent excitation in the near-ultraviolet wavelength region (350–400 nm).
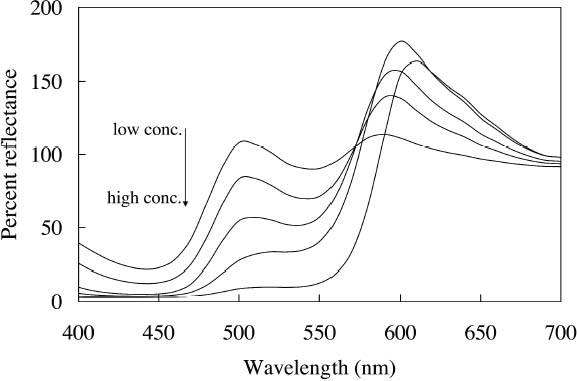
The multiple peaks in the fluorescent emission spectra of a number of watercolors (namely, Tropic Gold, Sunset Orange, Sunset Red, Ice Green, and Sunshine Yellow) are characteristic of mixtures of fluorescent dyes. These mixtures are formulated in a very specific way to take advantage of an interaction of the fluorescence between the components, in which the emission of one fluorescent dye is absorbed by a second dye, causing an increase in emission of the second dye. This so-called energy transfer from one dye to another may broaden, shift, and amplify the emission region of the fluorescent paint (Billmeyer and Hepfinger 1983; Christie 1993). 2.2 APPEARANCE CHANGES WITH WATERCOLOR CONCENTRATIONDaylight fluorescent watercolors are typically applied as washes, and their appearance is derived mainly from the absorption and fluorescence behavior, with very little optical scattering. Like paint glazes, then, fluorescent watercolors can experience shifts in hue at high concentration, as the absorption bands extend to a wider wavelength region in the reflectance spectrum (Johnston-Feller and Bailie 1982; Whitmore and Bailie 1997). In addition to the reflectance changes produced from very high absorption levels, fluorescent colorants may also fluoresce differently at high concentrations. Some fluorescence can be self-quenched—that is, a portion of the fluorescent emission can be absorbed by the colorant. Not only does self-quenching reduce the intensity of the fluorescent emission; it also leads to a shift of the peak fluorescence wavelength. An example of this quenching effect is the behavior of the Raspberry watercolor, whose reflectance spectra (measured using a xenon light source) of applications at different
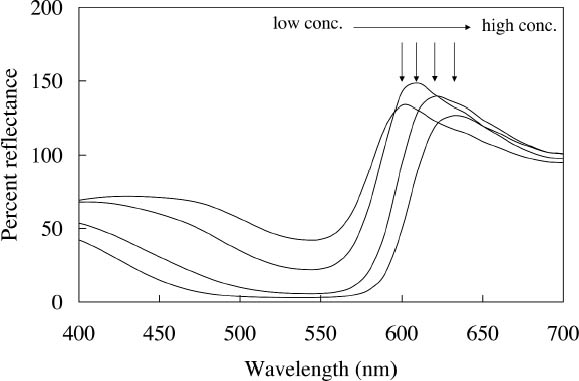
Concentration changes also have a profound effect on the reflectance spectra of those watercolors that experience energy transfer between their component dyes. An example is the Sunset Red watercolor, whose reflectance spectra (measured with the same xenon illumination described above) at different concentrations are shown in figure 4. At high concentrations the reflectance is dominated by the fluorescent emission at 600 nm from one of the two fluorescing components. As the concentration decreases, the energy transfer between the dyes becomes less efficient and the emission at 600 nm is greatly reduced, while the emission at 510 nm, from the other component, becomes apparent. For some watercolors, such as Sunshine Yellow, the relative contributions of the two fluorescing components are reversed upon dilution. This watercolor can shift from a reddish yellow when concentrated, with the dominant fluorescent emission at 600 nm, to a greenish yellow when dilute, when the 510 nm fluorescent emission dominates and the energy transfer to the 600 nm emitter almost disappears. These complex color changes with varied colorant concentration are obviously important to control in achieving the desired color effect during creation of a painting. These color changes may also occur as a fluorescent watercolor is degraded upon light exposure. The color changes that occur with fading can involve these same hue shifts accompanying dilute applications, increases or decreases in fluorescent emission, and the gradual bleaching of color to a lighter value. Such color changes can dramatically alter the original appearance of a work. These changes will also pose formidable challenges in color matching should light-induced fading affect the complicated interplay of chromatic color (from selectively absorbed wavelengths) and luminous color (from fluorescent emission) or disrupt the energy transfer in colorant mixtures and change the hue. These issues will be discussed at more length in section 4 below.
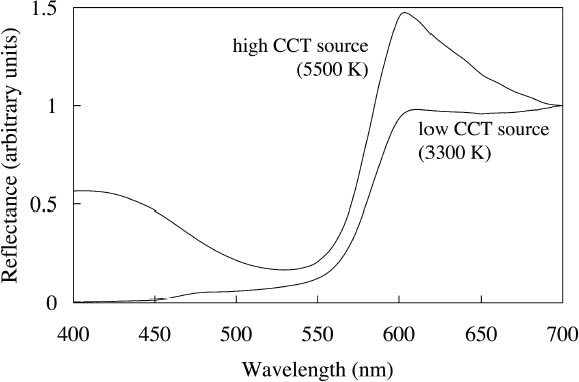
2.3 APPEARANCE UNDER DIFFERENT LIGHTING CONDITIONSThe fluorescent emission of such watercolors also affects the appearance of an application when viewed under different light sources in ways that may not be intuitive: changes in the spectral output of the illuminating source in one wavelength region can cause measurable changes in the reflectance at other parts of the spectrum. For example, one expects a color to look more yellow when viewing it under a low CCT source, such as an incandescent lamp. This illumination provides very low intensity at the blue end of the spectrum, so the spectrum of light reflected from an object under this source is also reduced at the blue wavelengths. A fluorescent watercolor under such a yellow source, however, not Conversely, illumination with lighting that has greater intensities at wavelengths that excite fluorescent emission will enhance the fluorescent appearance. While it is difficult to envision modified gallery lighting richer in blue-green wavelengths without many other color-rendering difficulties, the watercolors having substantial excitation in the near-ultraviolet region (noted above in section 2.1) would have their fluorescent appearance enhanced by supplementary illumination with near-ultraviolet black light sources. These watercolors may also have their fluorescent appearance enhanced by display under black lights alone (Tsang et al. 2004), in which case their appearance will be defined by their fluorescent emission spectrum only (since there is no perceptible reflected visible light under those conditions). Such display is likely to increase light-induced damage to the colorants, and it is more typical to consider restricting ultraviolet exposure to preserve the colors. The latter preservation strategy can usually be implemented with little concern about a negative impact on the appearance of an artifact. Yet for colorants exhibiting fluorescence excited by ultraviolet wavelengths, such a change in the lighting environment could affect the appearance of the object, reducing its chroma and luminosity, the key attributes that make it appear fluorescent.
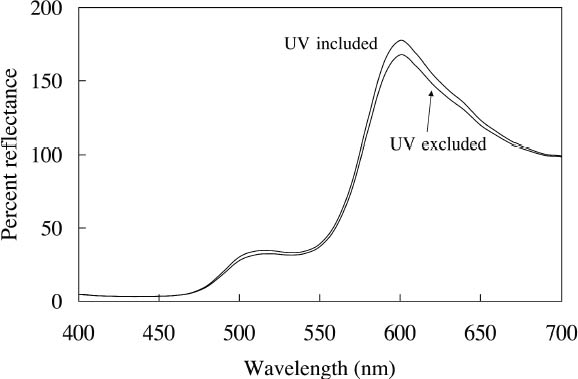
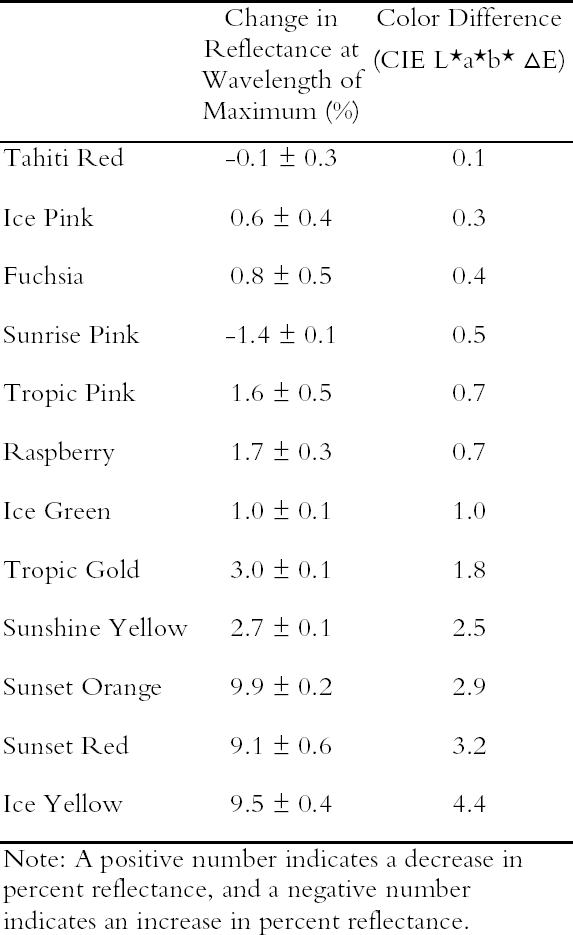
Because reducing ultraviolet exposure is an important preservation measure for many materials, including fluorescent colorants, it is critical to determine whether such a step would have an unacceptably large impact on the appearance of fluorescent watercolors. The difference in appearance for the Dr. Ph. Martin Radiant Concentrated watercolors when ultraviolet wavelengths are excluded from the illumination source has been measured, using a reflectance spectrometer in which the UV content of the probe beam (from a xenon lamp) could be either included or excluded. A typical result (in this case for Sunset Orange) is shown in figure 6, in which the prominent fluorescent emission peak at 600 nm is reduced only slightly when the ultraviolet wavelengths are excluded. This small (9.9%) decrease in total reflectance causes only a very small (2.9 Δ E units) change in the perceived color of this watercolor. Such a small change in fluorescent emission is reasonable because the excitation spectrum for this watercolor shows that the majority of fluorescent excitation occurs between 400 nm and 600 nm, with a relatively small amount of excitation from near-ultraviolet wavelengths. As shown in table 1, most of these daylight fluorescent watercolors
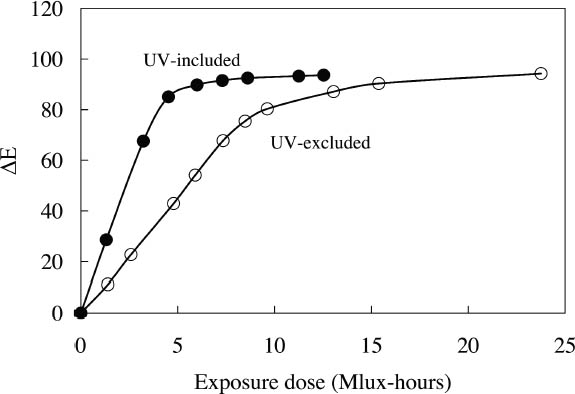
3 LIGHTFASTNESS UNDER DIFFERENT LIGHTING CONDITIONSMany daylight fluorescent colorants are known to be sensitive to damage from light exposure. In this study, the light sensitivity of the Dr. Ph. Martin watercolors was measured to assess the appearance changes that occur during fading and the rates at which fading occurs under light sources of different spectral output. Three different lighting conditions were explored: (1) a continuous high CCT source simulating daylight, which included near-ultraviolet wavelengths; (2) the same source with the near-ultraviolet wavelengths excluded; and (3) a black light source emitting only near-ultraviolet wavelengths. Low CCT light sources were not evaluated because it is unlikely that fluorescent materials would be exhibited under such sources.
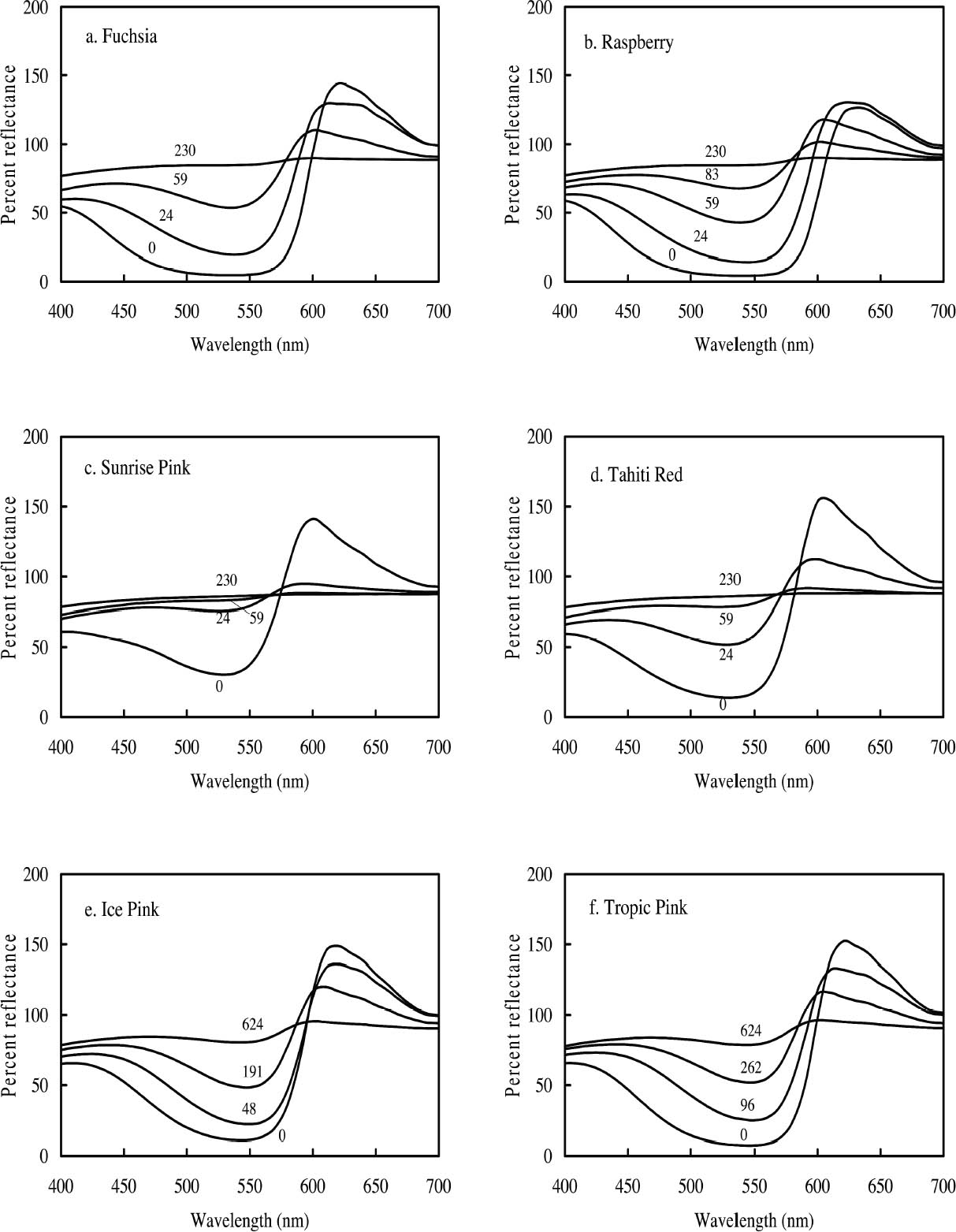
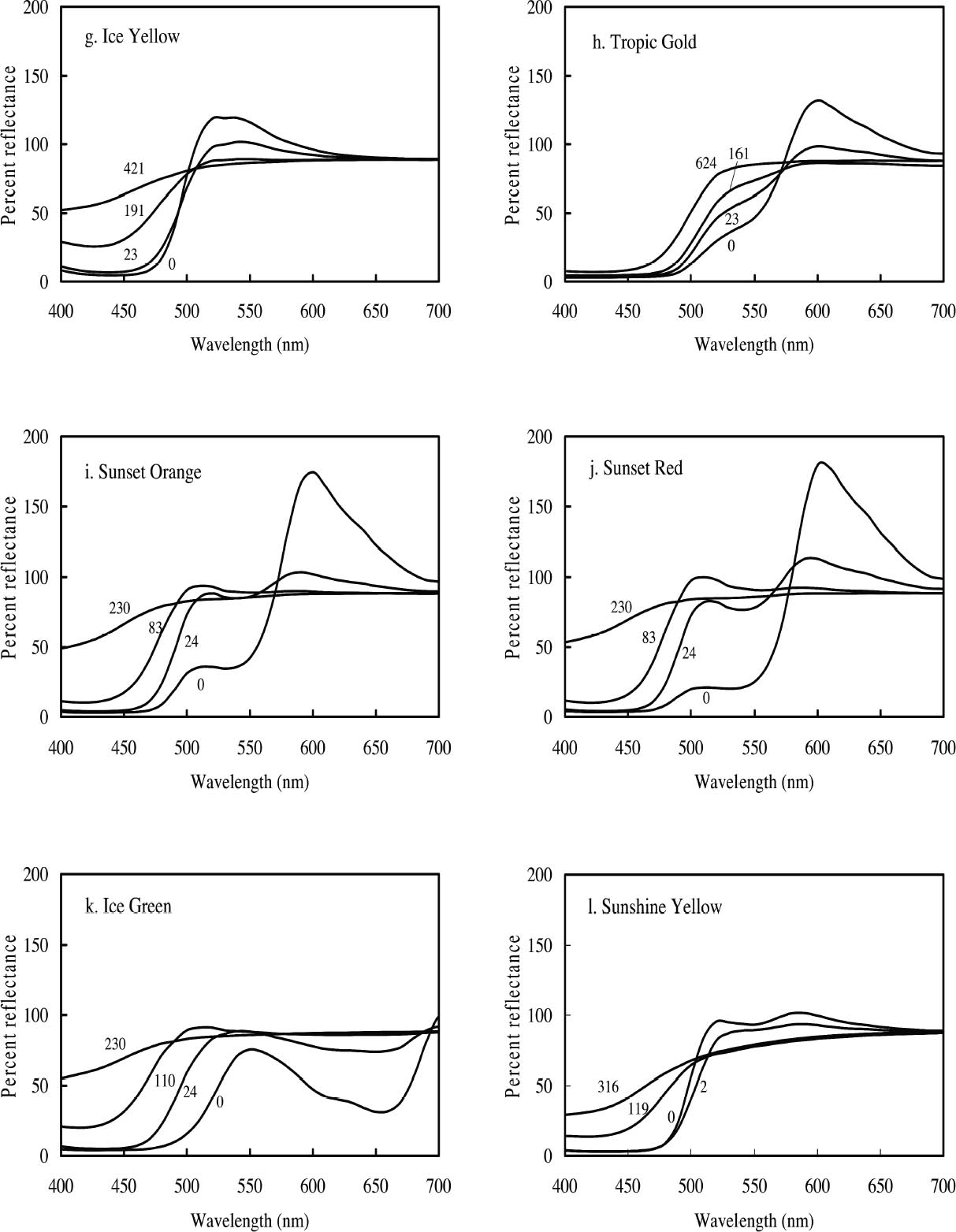
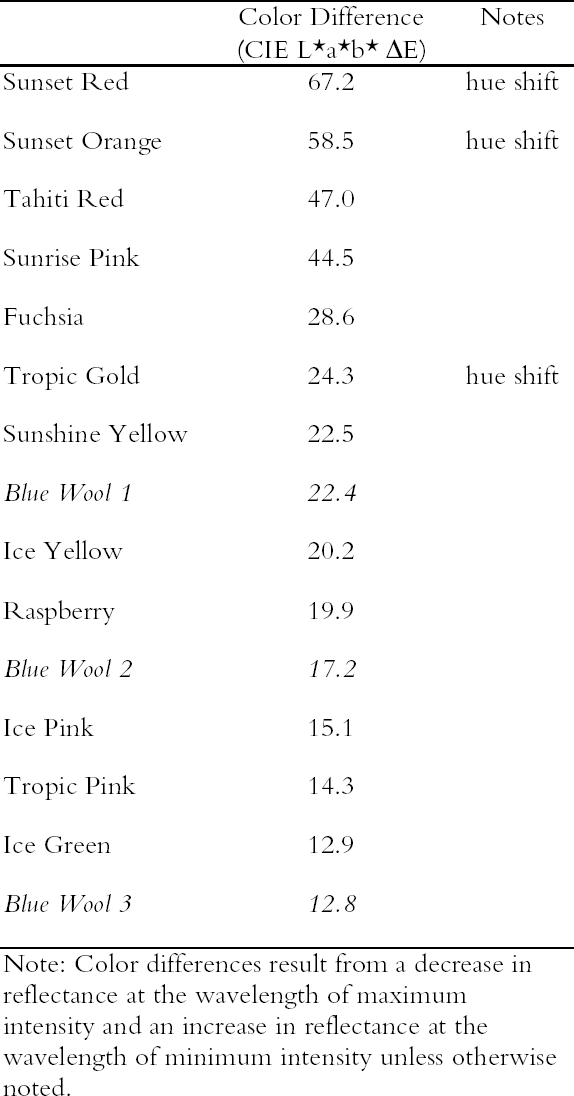
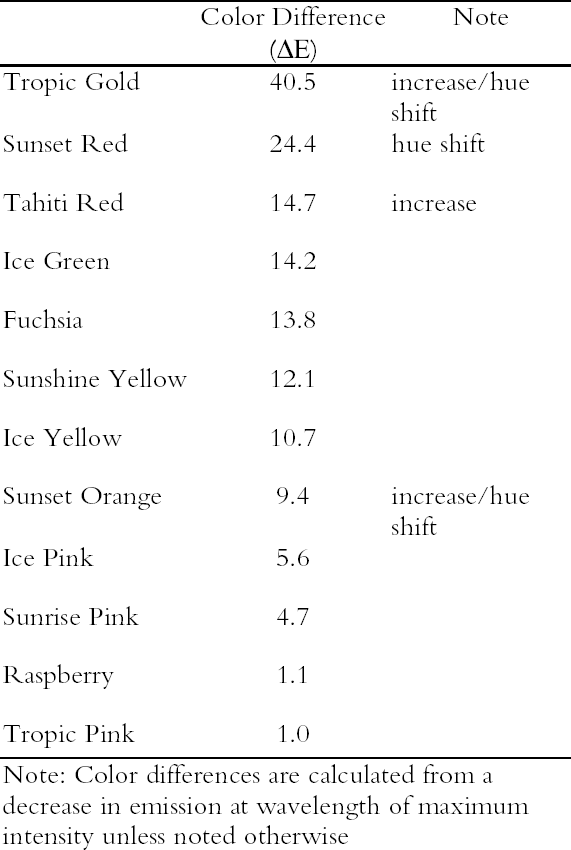
3.1 LIGHTFASTNESS UNDER DAYLIGHT CONTAINING UV3.1.1 Spectral and Appearance ChangesFigure 7 shows the reflectance spectra of the Dr. Ph. Martin watercolors at various intervals during their exposure to the output of a simulated daylight source (a xenon lamp), including near-ultraviolet wavelengths. These data show the spectral changes that occur during fading where three types of change are displayed. In 7 of the 12 watercolors (figures 7a-g) light exposure causes a gradual loss of fluorescent emission (decrease in the apparent reflectance of the most prominent reflectance peak), until in very faded samples the fluorescent emission is nearly extinguished and the reflectance reaches a value near 80%, that of the white paper support. This loss of fluorescent emission is likely to be the phenomenon that has been observed by conservators and described as the darkening of the paint from light exposure (Ellis et al. 2002; Baxter 2003). As the 7 watercolors that display this first pattern of fading lose their fluorescent appearance, they also lose their strong absorption band (in the 450–550 nm region for all watercolors except Ice Yellow, whose absorption band is between 400 nm and 500 nm). The fading of these watercolors progresses until reflectance values between 450 nm and 550 nm (400–500 nm for Ice Yellow) reach 80%, the value for the paper alone. Thus both the fluorescent emission and absorbance of the paints decrease during the light exposure: they become less luminous and lighten toward white as they fade. A different pattern of spectral change occurs during the exposure of four of the paints (Tropic Gold, Sunset Orange, Sunset Red, and Ice Green, This unusual spectral change during fading occurs in these paints because their original appearance depends on energy transfer between two fluorescing dyes in the paint, a process described above in section 2. In the unfaded paint, light is absorbed by one colorant (the donor) and emitted at around 510 nm, and the second colorant in the mixture (the acceptor) significantly absorbs that emission and re-emits it near 600 nm. Thus the paint primarily displays the fluorescent emission at 600 nm of the second dye. Prolonged light exposure interrupts this process, apparently by quickly destroying the very fugitive acceptor dye. The fading paint thus takes on the appearance of a watercolor containing only the donor dye, which emits its fluorescence at the lower 510 nm wavelength, and a pronounced shift in hue accompanies the gradual lightening of the color. The 12th watercolor included in this study, Sunshine Yellow (figure 7l), is similar to these last four paints in that it seems to contain a mixture of fluorescent colorants. However, the fading of this mixture does not seem to involve the initial complete loss of one component of the mixture. Rather, both fluorescent constituents seem to contribute emission to the reflectance spectrum, at 520 nm and at 590 nm, and light exposure causes both emission peaks to disappear essentially together. For this watercolor only, the fluorescent emission is first lost, and then the watercolor fades, seen by an increase in reflectance between 400 nm and 500 nm. In this case, even though there seems to be a mixture of colorants, the watercolor simply fades toward white without the abrupt shift in hue seen with the other mixtures. Upon visual inspection, all 12 of these watercolors eventually show a mottled and dull appearance after prolonged exposure to simulated daylight regardless of the type of change that occurs. This faded appearance helps to distinguish a light-damaged area of color from a fresh application of dilute color and may be useful when inferring the original appearance of an artwork, discussed further in section 4.2. 3.1.2 Fading RateThe fading rate of these paints was determined by calculating, from the measured reflectance spectra, the change in color as it was measured under a particular reference light source (the standard illuminant D65 was used). As the spectrum changed during fading, the difference in perceived color (Δ E) was derived using the 1976 CIE L*a*b* formula. The amount of color difference produced during the light exposure is a measure of the paint's lightfastness, and the observed fading rate was also compared to that of ISO Blue Wool Fading Standards exposed to the same light source. Table 2 shows the Δ E increase produced during the exposure of the watercolor samples and Blue Wool Standards to simulated daylight, including the near-ultraviolet wavelengths in the exposure. Most of these paints were very sensitive to light exposure, with a lightfastness less than or comparable to Blue Wool 1 or 2 in this exposure test. Only Ice Pink, Tropic Pink, and Ice Green had moderate light sensitivity, fading at rates comparable to Blue Wool 3. Table 2 also denotes when a hue shift experienced by a specific watercolor is responsible for the calculated color change (Δ E). The Δ E values for all other watercolors resulted from more typical fading behavior (i.e., lightening toward white). 3.2 PROTECTION GAINED FROM UV FILTRATIONRemoval of UV wavelengths from exhibition lighting conditions is a common museum practice designed to slow the rate of appearance change in
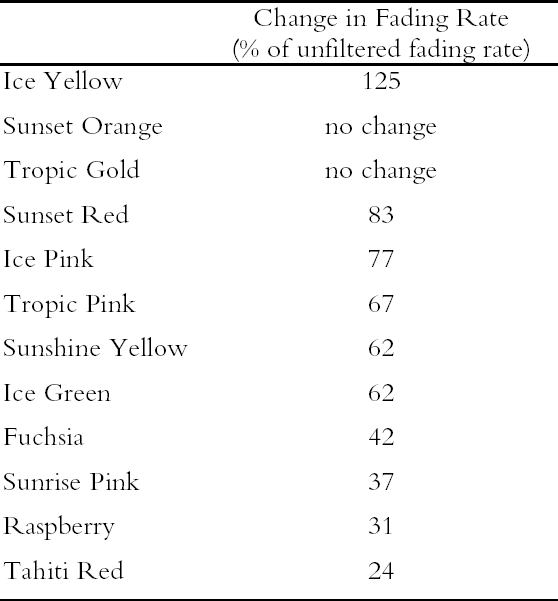
It is important to note that those watercolors showing the greatest preservation benefit from the removal of UV radiation are the same watercolors showing the least amount of appearance change from the removal of UV wavelengths (see section 2.3). In other words, for some of the watercolors studied, removing UV will slow their fading rate significantly with little perceptible change in appearance. 3.3 LIGHTFASTNESS UNDER A BLACK LIGHT SOURCE3.3.1 Spectral and Appearance ChangesOccasionally daylight fluorescent colorants will be exhibited under UV or black light alone. Therefore, the light sensitivity of the Dr. Ph. Martin watercolors when exposed to black light was also considered. The spectral changes produced during fading under these conditions were similar to those found when exposed to simulated daylight sources. Six of the 12 watercolors (Fuchsia, Raspberry, Sunrise Pink, Tahiti Red, Ice Pink and Tropic Pink)
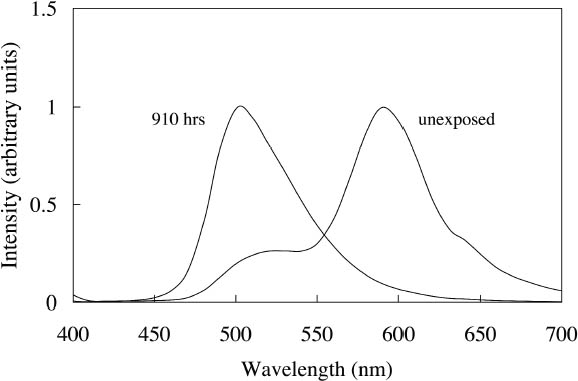
Ice Yellow and Sunshine Yellow experienced a slight shift in their fluorescent emission peaks near 500 nm toward shorter wavelengths as they faded. This shift can be accounted for by the change in colorant concentration experienced by the watercolors as they fade. As explained in section 2.2, the position of the fluorescent emission peak shifts toward shorter wavelengths with a decrease in concentration of the colorant, a change one expects as the colorant fades toward white. The last four watercolors (Tropic Gold, Sunset Orange, Sunset Red, and Ice Green) exhibited unexpected fading behavior from exposure to a black light source. The paints still experienced a loss of fluorescent emission at the longer wavelength emission peak, along with an increase in intensity of the shorter wavelength emission peak. This behavior was seen in the fading of dye mixtures in section 3.1.1, where the long wavelength emission peak was lost from destruction of the acceptor dye that disrupts the energy transfer. In this case, the emission peak at shorter wavelengths did not continue to fade further from black light exposure, as they had when exposed to simulated daylight. It seems that the dye emitting at shorter wavelengths (510 nm) is not rapidly degraded by UV. This behavior makes the hue shift experienced by Tropic Gold, Sunset Orange, and Sunset Red even more prominent. The spectra of light emitted from Sunset Red under black light illumination, before and after 910 hours of exposure, are shown in figure 9 as an example. The hue shifted from orangered to green and remained green throughout the remainder of the fading experiment. As a result, distinguishing among the appearance of Tropic Gold, Sunset Orange, Sunset Red, and other watercolors that exhibit this 510 nm emission peak (Ice Green, Ice Yellow, and Sunshine Yellow) when illuminated with black light would become very difficult after only a short exposure to those conditions.
3.3.2 Rate of Color ChangesThe fading rates of these watercolors were determined by calculating the color change (ΔE) as it would appear under black light illumination. The ΔE values for each watercolor are listed in table 4, and a description of the measurement and calculation used to determine ΔE values is given in the appendix below. These paints show significant light sensitivity under black light illumination. Many show a large change in appearance (ΔE > 10) after only a brief exposure. There is some difference in the light sensitivity of these watercolors when exposed to UV alone compared with results from their exposure to a daylight source (see table 2). Some of the watercolors (Tropic Gold, Tahiti Red, and Sunset Orange) experienced an increase in emission as a result of black light exposure. One possible explanation for this behavior is the presence of a nonfluorescent toner in the colorant that contributes only chromatic color to
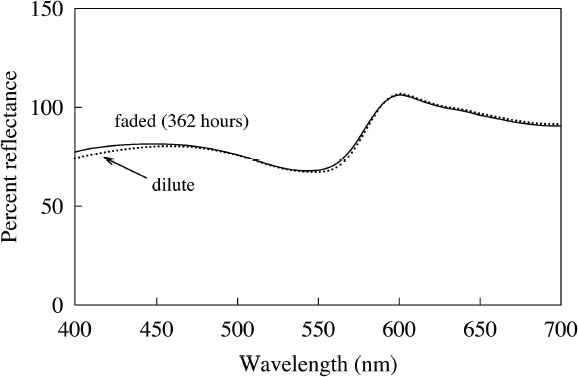
Figure 9 shows the spectral change experienced by Sunset Red, which occurs rapidly under black light conditions. The same hue shift from red to green fluorescent emission occurs for the Sunset Orange and Tropic Gold watercolors. Because of this prominent shift, the ΔE values (when viewed under black lights) for Tropic Gold, Sunset Red, and Sunset Orange following black light exposure are relatively high (see table 4), indicating a significant change in appearance. This shift in peak emission wavelength was also seen under simulated daylight exposure. However, under those conditions the short-wavelength emission peak (510 nm) continued to degrade, while under black light conditions the green fluorescent emission peak remained intense through the duration of the fading experiment. 4 CONSERVATION ISSUES4.1 COLOR COMPENSATION FOR FLUORESCENT PAINTSThe presence of fluorescent colors on a work of art poses some difficult restoration problems. Foremost among them is matching the luminosity and high chroma that are essential to fluorescent appearance when inpainting damaged areas. Six of the 12 watercolors—Fuchsia, Raspberry, Sunrise Pink, Tahiti Red, Ice Pink, and Tropic Pink—retain their fluorescent character throughout the fading process (see fig. 7). This finding is evident from the reflectance for each of these watercolors, which remains higher than the reflectance of the white paper alone (approximately 80%). It would be impossible to achieve even a metameric match to these colors using a nonfluorescent colorant. One could consider using paints that are identical to the paint on the original artwork to match the fluorescent appearance. For this strategy to be feasible, the retouching paint must match the appearance of a color that has already suffered some fading from light exposure. To see if a fluorescent watercolor could be used to match itself in a faded state, a range of colors were produced from dilutions of each watercolor. These colors were measured and compared to colors produced during fading. Figure 10 shows the spectra for both diluted and faded Tropic Pink. This reasonably good spectral match (ΔE = 0.65) is maintained through all applications of the color from high to low concentrations. This result is typical for the six watercolors mentioned above—Fuchsia, Raspberry, Sunrise Pink, Tahiti Red, Ice Pink, and Tropic Pink. Thus, the original
The final six watercolors—Ice Yellow, Tropic Gold, Sunset Orange, Sunset Red, Ice Green, and Sunshine Yellow—all have more complex fading behavior under daylight exposure (see fig. 7). All show complex spectral changes after short exposure times (approximately 24 hours), and some display significant shifts in hue as a result. Since such spectral shifts occur during fading, one might expect that color matching with the original unfaded colorant would be difficult. To assess whether color matches to these faded paints are feasible, the colors produced from dilutions of these six watercolors were measured and compared to their faded counterparts. The colors seem to match reasonably well when the concentration of color is high (i.e., only slightly faded). However, after more extensive fading, the diluted color is no longer able to match the faded appearance. It is apparent from the data shown in figure 7 that these watercolors lose their fluorescent character—luminosity and high chroma—during fading. As a result, nonfluorescent paints may be better choices for color compensation for these very degraded fluorescent paints. 4.2 INFERRING ORIGINAL APPEARANCEConservators and curators frequently strive to understand and present the original appearance of a work of art. The choice of lighting is often a critical factor in how a picture is perceived. For works containing fluorescent watercolors, that choice is particularly crucial because the degree of fluorescent appearance (chroma and luminosity) of these materials can be altered substantially depending on the choice of lighting environment. Aging of works containing fluorescent watercolors poses a different challenge in assessing the original appearance of a painting, for it is sometimes difficult to determine whether a fluorescent color was originally present. Some information about condition and possible alteration of the watercolors in this study can be gained from visual inspection. When viewed in daylight, faded areas of these colors appear mottled and dull. It is unlikely that one would confuse such faded areas with dilute applications (low color concentration) of the same watercolor, and one might in fact mistake such passages as applications of nonfluorescent paint. It can be confirmed that these colors are fluorescent by viewing them under black light. Significantly faded watercolors still appear to weakly fluoresce when exposed to ultraviolet radiation, even if they no longer appear remarkably luminous under daylight illumination. This weak fluorescent emission under black light illumination from obviously faded watercolor areas may indicate an original appearance of the watercolor that was darker and more fluorescent than its current appearance. After extensive fading, several of these watercolors—Ice Yellow, Tropic Gold, Sunset Orange, Sunset Red, Ice Green, and Sunshine Yellow— become indistinguishable from one another, all appearing dull yellow-brown in color. In this case it would be impossible by simple inspection to determine both the original depth of shade and the hue of the original color. Visual inspection of these watercolors becomes more difficult when viewed under black light. Under these conditions one observes only the fluorescent emission, and the visual cues that distinguish these colors as faded (mottled and dull appearance) are not visible. Unfortunately, without these visual cues or without some knowledge of the color's original appearance and fluorescent intensity, it is difficult 5 CONCLUSIONSThe appearance of the fluorescent watercolors in this study was found to be dependent on the concentration of the paint application. As with glazes, dark applications of these watercolors (i.e., high concentrations of color) will experience a shift in hue due to the widening of their absorbance band. They may also experience self-quenching, which results from the absorbance of fluorescent emission by the colorant itself and further alters the apparent hue and chroma. Some watercolors (Tropic Gold, Sunset Orange, Sunset Red, Ice Green, and Sunshine Yellow) experience significant spectral shifts at high concentrations because of the process of energy transfer between two dyes used to create each of these watercolors. As one should expect, the appearance of these watercolors is also dependent on the light source used to illuminate them. High CCT light sources (i.e., simulated daylight) contain the appropriate wavelengths to produce both reflected and luminous color. Low CCT sources (i.e., incandescent lamps) will not produce a luminous appearance because they do not contain the appropriate wavelengths to excite fluorescent emission. Black light sources will produce the luminous appearance from the fluorescent emission alone. Removal of near-ultraviolet wavelengths from a simulated daylight source—a common preservation measure—does not significantly alter the appearance of the watercolors studied. Designed as daylight fluorescent watercolors, they are primarily excited by visible wavelengths, and the removal of UV wavelengths does not significantly reduce the intensity of their fluorescent emission. Nine of the 12 watercolors studied showed light sensitivities comparable to Blue Wool 1 and Blue Wool 2 when exposed to simulated daylight. Only Ice Pink, Tropic Pink, and Ice Green showed moderate light sensitivity comparable to Blue Wool 3. Removal of UV wavelengths slowed the fading rate for several watercolors—Sunset Red, Ice Pink, Tropic Pink, Sunshine Yellow, Ice Green, Fuchsia, Sunrise Pink, Raspberry, and Tahiti Red—to 24–83% of the rate under unfiltered daylight. Three types of change occurred to these watercolors during prolonged exposure to simulated daylight. Seven of the 12 watercolors tested (Fuchsia, Raspberry, Sunrise Pink, Tahiti Red, Ice Pink, Tropic Pink, and Ice Yellow) faded by a simultaneous loss in the ability to fluoresce and absorb light as the colorant was destroyed. Four other watercolors (Tropic Gold, Sunset Orange, Sunset Red, and Ice Green) lost one fluorescent emission peak (at longer wavelengths), while a second emission peak emerged at shorter wavelengths. This change occurred because of a disruption in energy transfer between two dyes (from the more rapid destruction of the acceptor dye) in the colorant mixture and resulted in a significant shift in hue as the watercolor faded. The 12th watercolor (Sunshine Yellow) also seemed to contain a mixture of fluorescent dyes; however, both dyes lost their ability to fluoresce simultaneously instead of showing the loss of one emission peak followed by the emergence of another. Prolonged exposure to black light produced fading results similar to those found upon exposure to simulated daylight. However, three of the watercolors (Tropic Gold, Tahiti Red, and Sunset Orange) experienced an increase in emission as a result of black light exposure. Also, the colorant component responsible for the green fluorescent emission (at 510 nm) found in several watercolors (Tropic Gold, Sunset Orange, Sunset Red, Ice Green, Ice Yellow, and Sunshine Yellow) was relatively stable to black light exposure. Because they all degrade to the same fluorescent green residue, it is difficult to distinguish the actual color of these watercolors after only a slight degree of fading under black light exposure. Matching the color of faded fluorescent watercolors with the original paint was possible in some cases. The spectra for 6 of the 12 watercolors (Fuchsia, Raspberry, Sunrise Pink, Tahiti Red, Ice Pink, and Tropic Pink) matched well at both high (slightly faded) and low (severely faded) color concentrations. Dilute applications of the other six watercolors (Ice Yellow, Tropic Gold, Sunset Orange, Sunset Red, Ice Green, and Sunshine Yellow) did not match their faded counterparts because the faded watercolor had experienced a significant loss of fluorescent emission. For these six, compensation of faded passages may be better achieved with paints containing nonfluorescent colorants. NOTES1. Two lamp types can be used as black light sources. Both are UVA-emitting lamps with peak intensity at 365 nm in the UV and narrow energy peaks at 440 nm and 550 nm in the visible region. One lamp type retains the visible light intensities, and the other filters the intensities above 400 nm using a dark blue filter. The latter of these two lamps was used for the black light experiments in this study, so the behavior of these watercolors under UV wavelengths alone could be explored. 2. The change in fading rate was calculated from the slope of the Δ E curves for both UV-included and UV-excluded simulated daylight exposure between 0 and 1.3 Mlux-hours. The ratio of the slope of the UV-excluded curve to the slope of the UV-included curve was multiplied by 100 to give the percent of unfiltered fading rate. APPENDIXAPPENDIX: EXPERIMENTALThe 12 fluorescent watercolors (Fuchsia, Raspberry, Sunrise Pink, Tahiti Red, Ice Pink, Tropic Pink, Ice Yellow, Tropic Gold, Sunset Orange, Sunset Red, Ice Green, and Sunshine Yellow) from the Dr. Ph. Martin Radiant Concentrated Water Color line of products were studied. Samples were prepared by applying each watercolor, by brush, to Arches hot press watercolor paper and then allowing them to dry. Seven replicate samples were prepared for exposure to various lighting conditions (described below). Multiple applications of the watercolor were made when necessary to ensure that replicates had equivalent initial depths of shade. Samples with a range of color concentrations (low to high) were prepared by application of diluted watercolor paint to paper. Pairs of replicate samples were exposed to each of the following accelerated aging conditions: (1) xenon lamp exposure with UV included, (2) xenon lamp exposure with UV excluded, and (3) black light (F20T12/BLB from General Electric). UV-absorbing Plexiglas (UF3) sheets were used to filter out the UV from the xenon lamp source when necessary. A Heraeus Suntest CPS, which employs a xenon source filtered to eliminate infrared and short wavelength ultraviolet, was used for light exposure. Samples were placed on a watercooled sample tray held at 20�C with recirculating chilled water. The intensity of light in the Suntest CPS, measured by a radiometer in lux for a diffuse light source (International Light IL1700 with detector SED010 351, filter Y 70, and diffuser W 323), was 5.45 � 104lux with UV included in the output and 4.96 � 104lux with UV excluded from the output. The intensity under black lights, measured by a radiometer for total ultraviolet radiation (International Light IL1700 with detector SED400 383, filter 20162, and diffuser W315) was 1.63 � 10-3 W/cm2. Aging experiments were also conducted under either high-output daylight fluorescent lamps (General Electric F48T12-D-HO) or facing a north window, with similar results obtained. Following exposure under accelerated light-aging conditions, some reversion of the faded fluorescent color was observed if the samples were allowed some period of dark storage prior to color measurement. For consistency, color measurements were made immediately after samples were removed from their exposure conditions. These data were compared to naturally aged samples (daylight exposure through a north-facing window). The data from artificially aged samples were consistent with the natural aging result. However, only the results of the artificial aging tests are reported. Color measurements, for samples exposed to simulated daylight conditions, were made using a GretagMacbeth Color Eye 7000 Colorimeter. The reflectance data collected by this instrument (specular reflectance excluded) include both the directly reflected light and the emitted fluorescence. A UV filter was placed in the optical path before the light reached the sample to measure appearance changes both with and without UV wavelengths. Optiview version 1.5 software for this instrument provided reflectance data as well as tristimulus values calculated for 2� observer and standard illuminant D65. Color differences were calculated with the 1976 CIE L*a*b* formula. The spectra of the fluorescent emission under black light illumination were measured directly, without reference to a white standard, using a fiberoptic-based photodiode array spectrophotometer (Control Development Model PDA-512-USB). Under these conditions, the emission spectra of some of the watercolor samples (Raspberry, Tahiti Red, Tropic Pink, Ice Pink, Fuchsia, and Sunrise Pink) were so weak that the spectra displayed artifacts from the black light and the optical brighteners in the paper support. These artifacts were removed from the spectra by setting the intensities in the 400–515 nm region to the value Reflectance measurements used to determine appearance under light sources with different corre-lated-color-temperatures (CCT) were also made with the fiberoptic-based spectrophotometer, using a yellow filter (Schott Glass Technologies FG-13) to lower the CCT of the xenon lamp used as the illumination source. The measured intensities were normalized to 1.0 at 700 nm (a wavelength that is not likely to be affected by the change in CCT) after collection. Fluorescence excitation and emission spectra were collected using a Fluorolog ISA (Jobin Yvon, Horiba Group). Samples on watercolor paper were cut into 4 cm � 13 mm strips and placed inside a quartz fluorescence cuvette for analysis. The paper samples were positioned so that the exciting light beam was directed at about a 45δ angle to the paper surface, and the emitted fluorescence was collected at about a 45� angle. A complete list of experimental conditions can be found in table 5. ACKNOWLEDGEMENTSThis work was performed at the Art Conservation Research Center at Carnegie Mellon University and was supported by a grant from the Andrew W. Mellon Foundation. The authors would like to thank Dr. Susan Daly and Dr. Robert Tilton of the Biomedical Engineering Department at Carnegie Mellon University for the use of their Fluorolog ISA fluorimeter.
REFERENCESBaer, N. S., A.Joel, R. L.Feller, and N.Indictor. 1986. Indian yellow. In Artists' pigments: A handbook of their history and characteristics. Vol. 1, ed. R. L.Feller. Washington, D. C.: National Gallery of Art. 17–36. Baxter, E.2003. Personal communication. Carnegie Museum of Art, Pittsburgh, Pa. Berns, R. S.2000. Billmeyer and Saltzman's principles of color technology. 3rd ed. New York: John Wiley and Sons. Billmeyer, F. W., and L. B.Hepfinger. 1983. Energy transfer between fluorescent organic pigments. Color Research and Application8(1):12–16. Christie, R. M.1993. Fluorescent dyes. Review of Progress in Coloration23:1–18. Dane, C.1977. Fluorescent colourants and their use in printing inks. British Ink Maker20(1):11–13. de la Rie, R.1982a. Fluorescence of paint and varnish layers. Part 1. Studies in Conservation27:1–7. de la Rie, R.1982b. Fluorescence of paint and varnish layers. Part 2. Studies in Conservation27:65–69. Ellis, M. H., C. W.McGlinchey, and E.Chao. 2002. Daylight fluorescent colors as artistic media. In The broad spectrum: Studies in the materials, techniques, and conservation of color on paper, ed. H. K.Stratis and B.Salvesen. London: Archetype Publications. 160–66. Johnston-Feller, R.2001. Color science in the examination of museum objects: Nondestructive procedures. Los Angeles: Getty Conservation Institute. Johnston-Feller, R., and C.Bailie. 1982. An analysis of the optics of paint glazes: Fading. In Science and technology in the service of conservation, ed. N. S.Brommelle and C.Thomson. London: IIC. 180–85. Meadows, W.1974. Formulating with fluorescent pigments. Paint and Varnish Production64(9):31–37. Smith, T.1982. Luminescent and fluorescent pigments for printing inks. Polymers Paint and Colour Journal172(4077):542, 545–46. Tsang, J., S. E.Pinchin, K.Almond, and C. S.Tumosa. 2004. Conservation of murals in the Alameda theatre: Reviving former cutting-edge fluorescent paint and black-light technology. In Modern art, new museums, ed. A.Roy and P.Smith. London: IIC. 185–88. Voedisch, R. W.1973. Luminescent pigments, organic. In Pigment handbook, Vol. 1, Properties and economics, ed T. C.Patton. New York: John Wiley and Sons. 891–903. Whitmore, P. M., and C.Bailie. 1997. Further studies on transparent glaze fading: Chemical and appearance kinetics. Journal of the American Institute for Conservation36:207–30. FURTHER READINGLakowizc, J. R.1999. Principles in fluorescence spectroscopy. 2nd ed. New York: Kluwer Academic/Plenum. Lower, E. S.1996. Fluorescent paints and pigments. Pigment and Resin Technology25(3):15–18. Yoshizawa, A.2000. Daylight fluorescent pigments in works of art: Properties, history and fading. Master of Art Conservation thesis, Queen's University, Kingston, Ontario. SOURCES OF MATERIALSDr. Ph. Martin Radiant Concentrated WaterColors Dick Blick Art Materials P.O. Box 1267 Galesburg, Ill. 61402-1267 (800) 828-4548 www.dickblick.com Arches hot press watercolor paper, 160 lb.Utrecht Art Supplies 1930 East Carson St. Pittsburgh, Pa. 15203 (412) 432-1945 Colorimeter (Color Eye 7000)GretagMacbeth 617 Little Britain Rd. New Windsor, N.Y. 12553 Jobin Yvon Inc. Horiba Group 3880 Park Ave. Edison, N.J. 08820 (732) 494-8660 www.jyhoriba.com Photodiode array spectrophotometer (ModelPDA-512-USB) Control Development, Inc. 2633 Foundation Dr. South Bend, Ind. 46628 (574) 288-7338 www.controldevelopment.com Light exposure apparatus (Heraeus Suntest CPS)Atlas Electric Devices Co. 4114 North Ravenswood Ave. Chicago, Ill. 60613 (773) 327-4520 Black lights (General Electric modelF20T12/BLB) Grainger, Inc. 3150 Liberty Ave. Pittsburgh, Pa. 15201-1416 (412) 281-4477 www.grainger.com 75W xenon arc lamp (cat. no. 6251)Spectra-Physics, Inc. 150 Long Beach Blvd. Stratford, Conn. 06615 (203) 377-8282 www.spectra-physics.com Blue Wool reference cardsTalas 20 West 20th St., 5th Floor New York, N.Y. 10011 (212) 219-0770 www.talasonline.com Radiometer (model IL1700)International Light, Inc. 17 Graf Rd. Newburyport, Mass. 01950-4092 (978) 465-5923 www.intl-light.com Low CCT filter (FG-13)Schott Glass Technologies, Inc. 400 York Ave. Duryea, Pa. 18642 (570) 457-7484 www.us.schott.com AUTHOR INFORMATIONSANDRA A. CONNORS-ROWE received a BS in chemistry from Wayne State University. During this time she gained experience in collections management at the Henry Ford Museum in Dearborn, Michigan, and as a painting conservation technician at Conservation and Museum Services in Detroit. Following her BS degree, Connors-Rowe received a master of art conservation degree with a concentration in conservation science from Queen's University in Kingston, Ontario. Since 1998, she has been a conservation scientist with the Art Conservation Research Center at Carnegie Mellon University. Her research has been directed toward noninvasive methods for evaluating the degradation of materials. Address: Carnegie Mellon University, 700 Technology Dr., Pittsburgh, Pa. 15219. E-mail: scon-nors@andrew.cmu.edu HANNAH R. MORRIS received a PhD in analytical chemistry from the University of Pittsburgh, where her research focused on materials characterization in complex polymer blends using spectroscopy and chemical imaging techniques. Since 2000 she has been deputy director of the Art Conservation Research Center. Address as for Connors-Rowe PAUL M. WHITMORE received a PhD in physical chemistry from the University of California at Berkeley. Following an appointment at the Environmental Quality Laboratory at Caltech studying the effects of photochemical smog on works of art, he joined the staff of the Center for Conservation and Technical Studies at Harvard University Art Museums. Since 1988 he has been director of the Art Conservation Research Center at Carnegie Mellon University, where his research has been directed toward the study of the permanence of modern art and library materials. Address as for Connors-Rowe
 Section Index Section Index |