A METHOD FOR THE AQUEOUS DEACIDIFICATION OF OXIDIZED PAPERJOHN BOGAARD, HANNAH R. MORRIS, & PAUL M. WHITMORE
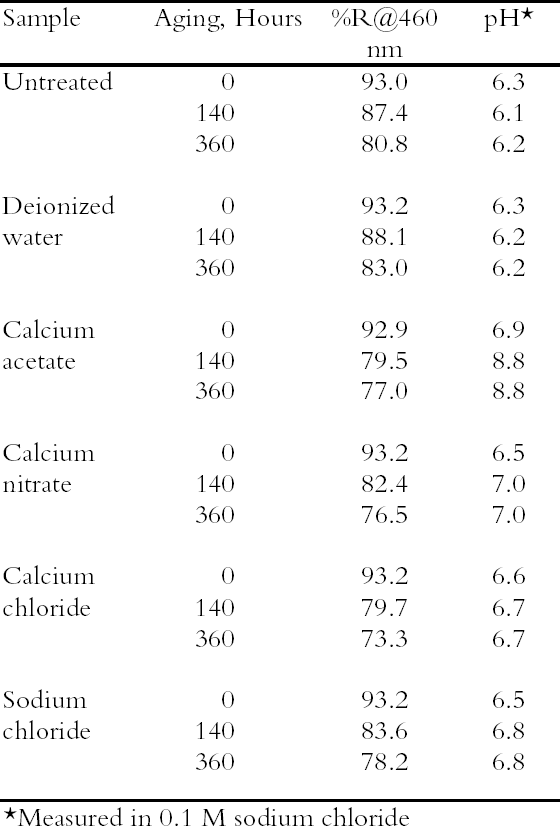
3 RESULTS AND DISCUSSION3.1 SURVEY OF CONCENTRATED SALTSPhoto-oxidized papers were immersed in concentrated (0.1 M) solutions of four different salts as well as deionized water, and the unrinsed sheets were thermally aged. The results are summarized in table 2; in this case only pH and brightness were tracked. The oxidized paper was only mildly acidic (pH = 6.3), and all the salt solution treatments raised the pH almost to neutrality, while the water treatment did not change the pH. Upon thermal aging, the pH of the treated sheets rose. This effect was small for three of the salts but much higher for the calcium acetate. In this case, it may be that, at the high temperature and humidity of thermal aging, the excess salt decomposed to form acetic acid that then vaporized, leaving an alkaline residue. It was decided not to examine this salt further. The reflectance data show greater yellowing of the salt-containing sheets upon thermal aging compared with the untreated or water-treated samples. Yellowing has been seen frequently with magnesium salt treatments (Bansa 1998), but it has been found in fewer instances with calcium salts (Hey 1979; Tang 1981). It is not clear what causes this effect or to what extent it would occur if the samples were held at room temperature. Nonetheless the effect is undesirable, and ways to minimize it were explored in the following experiments. All the salts surveyed deacidified the paper samples to a satisfactory degree, and all the treated sheets yellowed upon thermal aging. Since there was no substantial difference between them, calcium chloride was chosen for further examination as a possible conservation treatment, due primarily to its inert nature and high solubility.
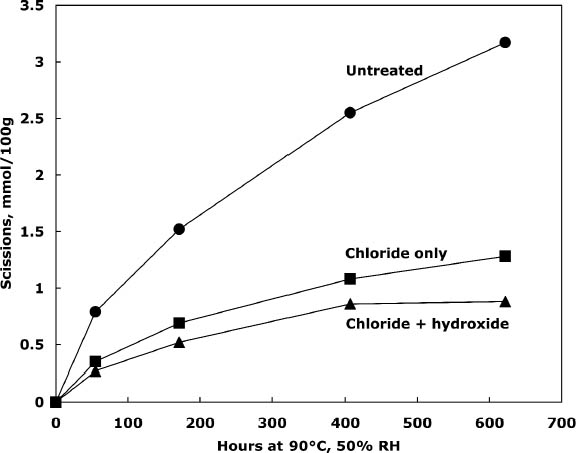
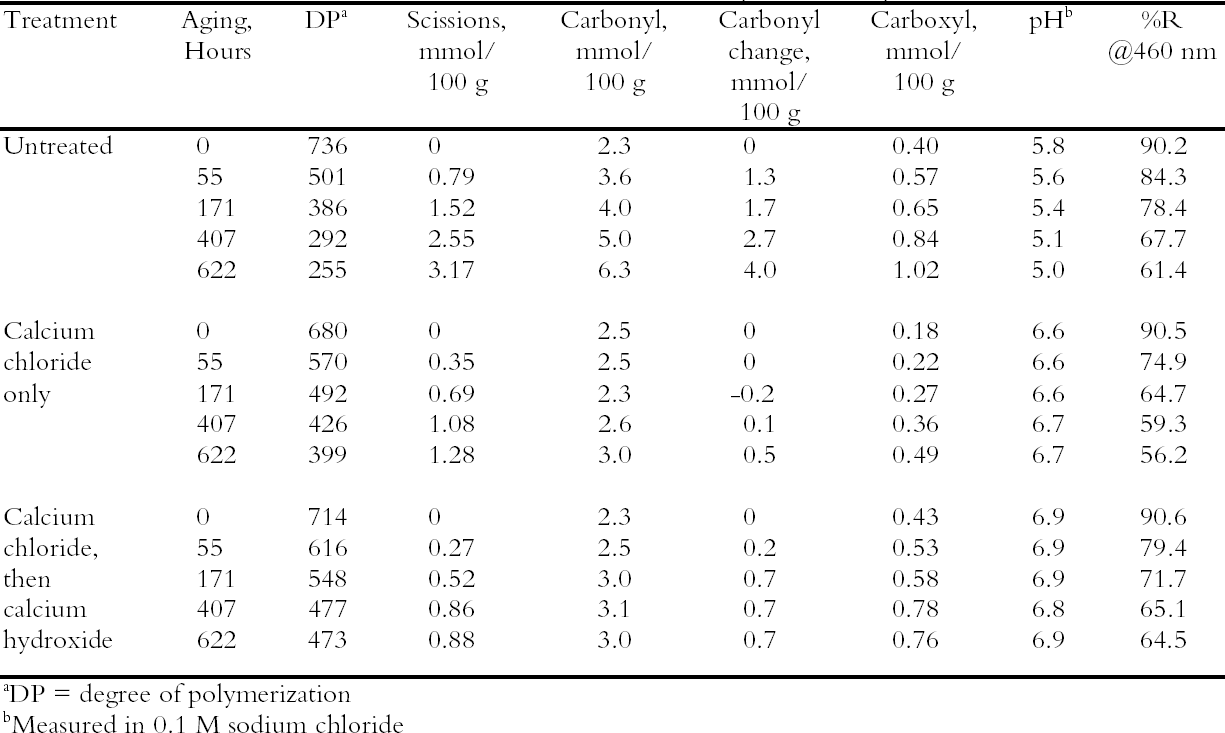
3.2 TREATMENT WITH CALCIUM CHLORIDE ALONE OR WITH CALCIUM HYDROXIDEPhoto-oxidized sheets treated with calcium chloride alone were compared with samples that were treated and then rinsed once with dilute calcium hydroxide to remove excess salt and give an alkaline reserve. The results of the treatment and subsequent thermal aging are collected in table 3. The treatment with chloride only raised the pH by almost a full unit before thermal aging, while the two-step treatment raised it even more. There was a slight reduction in DP after both treatments, which is not thought to be significant. The carbonyl group content was virtually unchanged for the two-step treated sample and slightly higher for the chloride-only treatment (probably due to its lower DP). The carboxyl content was also virtually unchanged for the two-step sample. The reduction of measured carboxyl groups with the chloride-only treatment was probably due to interference with the methylene blue test by the high amount of residual calcium in the sheet rather than an actual loss of these groups (Davidson 1948). The thermal aging results are graphed in figure 1 in terms of the cellulose scissions, or chain breaks, versus the hours of aging. The untreated paper deteriorated quickly, while the treatments greatly reduced this rate. The treatment with chloride only slowed the degradation by about two to three times, while the two-step treatment slowed it about three to four times. Referring back to table 3, the untreated sample showed a steady drop in pH over the aging period, while the carboxyl content grew significantly. For the two-step samples, the pH was unchanged, despite the increase in carboxyls,
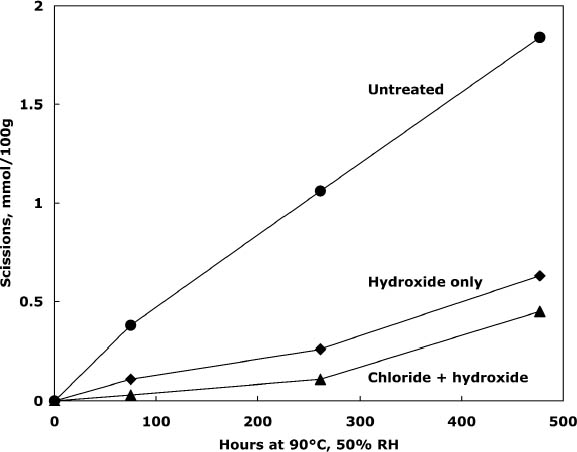
Comparison of the scissions with the change in carbonyl content shows an approximately equal ratio, indicating that the degradation was primarily due to acid hydrolysis, as expected when paper is thermally aged. It is unusual that the samples treated with chloride only did not seem to change in their carbonyl content at first despite their deterioration; every break in the cellulose chain should produce a new reducing end that is measured as a carbonyl group. It is not clear if the chain-breaking chemistry is altered for those samples that have a high amount of residual salt or if the excess salt is interfering with the carbonyl measurement in some way. The reflectance measurements show that the treated samples yellowed faster initially than the untreated paper, even though the treated papers were chemically less degraded. However, the rate of darkening for these sheets gradually slowed as the aging progressed, unlike the untreated papers, which continued to darken steadily. The rinse of the paper after the concentrated salt treatment seemed to improve the darkening behavior, so more thorough rinsing was performed in the next experiment to see if the problem could be eliminated. 3.3 COMPARISON OF THE TWO-STEP TREATMENT TO CALCIUM HYDROXIDE ALONEPhoto-oxidized sheets were thermally aged before treatment to create more acidic, deteriorated samples that would pose a more difficult challenge to treat. After treatment with concentrated calcium chloride, the sample papers were rinsed twice with dilute calcium hydroxide to thoroughly remove excess salt and leave an alkaline reserve. Comparison was made with samples that were simply rinsed twice with dilute calcium hydroxide. The results of treatment and subsequent thermal aging are contained in table 4. The untreated samples had a pH of 5.6, while the pH of the treated sheets was raised to 6.8 in both cases. Both treatments showed a very slight reduction in DP coupled with a slight increase in carbonyl groups. There was virtually no change in the carboxyl content; rinsing after the concentrated salt treatment apparently eliminated the interference with the methylene blue test. The degradation of the samples upon thermal aging is shown in figure 2. Both treatments retarded the deterioration with the two-step treatment, slowing the degradation rate by four to five times and the hydroxide-only treatment, slowing it by about three to four times. These results were reflected in
The results of these last two experiments show that combining the two treatments for neutralization and alkalization worked better than either treatment alone in slowing degradation upon thermal aging, and they caused virtually no chemical damage. The concentrated calcium chloride solution seems to quickly draw out a significant amount of acidity from paper fibers, leaving the calcium hydroxide treatment better able to complete the deacidification process and create an alkaline reserve. However, it may also be desirable to stabilize or bleach oxidized papers with a reduction treatment. The drawbacks of using sodium borohydride to reduce the oxidation have been noted earlier; neutralizing the paper first should make this treatment more efficient and lower the risk of damage from both the alkalinity and the gas bubbles. A treatment sequence of neutralization of an acidic, oxidized paper with the concentrated salt solution, followed by reduction of the oxidation and then an alkaline rinse, should decrease the risks of these treatments while enhancing their beneficial effects. In the next section this sequence is demonstrated. 3.4 NEUTRALIZATION, REDUCTION, AND ALKALIZATIONThe oxidized, naturally aged paper was given a seven-step sequential treatment that included neutralization with concentrated calcium chloride solution, two rinsing steps, reduction with sodium borohy-dride solution, two more rinsing steps, and final deacidification with dilute (0.4 mM) calcium hydroxide solution. During the immersion of sample papers in the borohydride reduction step, it was observed that only a small amount of tiny bubbles formed on the neutralized sheet, which indicated that the decomposition of sodium borohydride was minimal. This result created a safer situation for the paper with regard to the bubbling and presumably a more efficient reduction treatment.
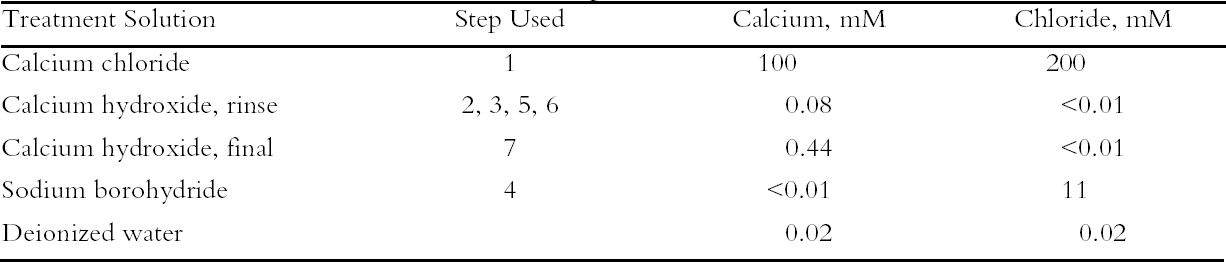
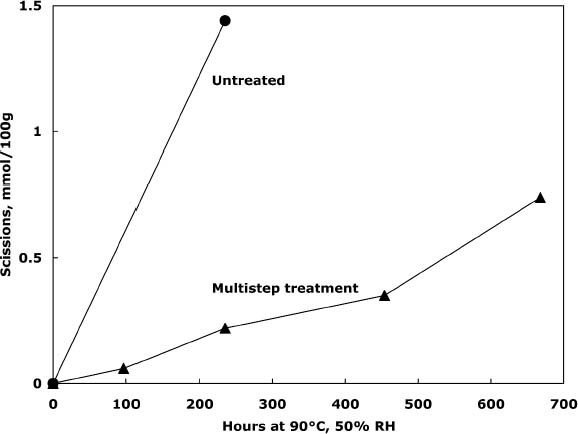
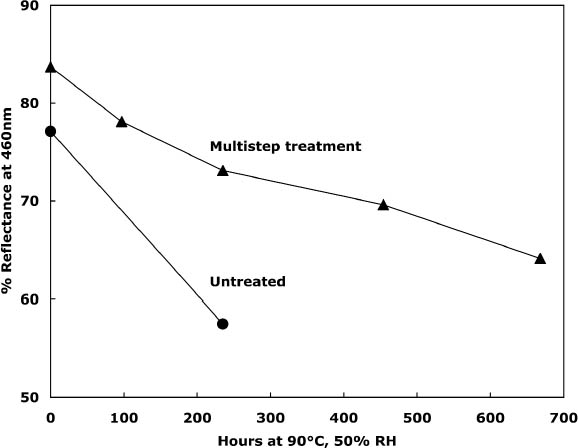
To characterize the treatment solutions more fully, the concentrations of calcium and chloride ions were measured using ion-selective electrodes. The data, presented in table 5, show the concentrations for the calcium chloride solution that were expected. The dilute calcium hydroxide solution is less concentrated, at 0.44 mM, than the estimation of 0.63 mM that was based on dilution of a saturated solution. Despite excess calcium hydroxide in the solution, full saturation apparently was not achieved. The solution of sodium borohydride had a surprisingly high amount of chloride impurity. The deionized water concentrations are presented for comparison and, as expected, had only trace amounts of the ions. The results of the chemical tests after treatment and thermal aging are found in table 6. The untreated sheet was fairly degraded with a pH of 4.5. The multi-step treatment raised the pH by almost two units to 6.4, while the DP was virtually unchanged. The lower value of the carbonyl content in the treated sheet roughly corresponded to reduction of all the oxidation groups on the cellulose chain. Calculations suggest that the remaining carbonyls may be the end groups of the chains, which are slower to react (Head 1955). The degradation of the samples upon thermal aging is shown in figure 3. (Due to the limited amount of these samples, only one untreated sheet was included in thermal aging for comparison.) The treated samples degraded about seven times more The brightness measurements (%R) indicate an increase in the reflectance of the sheets after treatment; this bleaching should be due to the sodium borohydride treatment (Tang 1986). The yellowing upon thermal aging is graphed in figure 4, and it can be seen that the rate of discoloration was much lower for the treated sheets than for the untreated. Measurements of the calcium and chloride ion contents in the treated sheets are included in table 6. The measurements are presented in units of mmol/100 g for comparison to the oxidation tests. (For comparison with other published work, these amounts are equivalent to 250–280 ppm of calcium in the treated sheets.) The treatments raised the calcium content to 0.68 mmol/100 g, which is a little lower than the carboxyl content of 1.00 mmol/100 g. The calcium ion is capable of binding with two carboxyl groups if they are in close proximity, so it is not known if all the carboxyls have been converted to the salt or if some remain in the acid form. However, the pH measurements imply that this paper is not fully neutralized. The chloride content was lowered slightly by this treatment sequence. The thorough rinsing with calcium hydroxide seemed to remove any chloride ions introduced by the concentrated salt treatment. With thermal aging the chloride content dropped in both the treated and untreated sheets. It is not clear if the high temperature and humidity conditions caused the ion to bind to something in the paper, forming an insoluble salt, or to react in another, unknown way. The amount of calcium in the sheets remained about the same upon aging; the variation between samples is probably due to differences in pickup during treatment.
|