A METHOD FOR THE AQUEOUS DEACIDIFICATION OF OXIDIZED PAPERJOHN BOGAARD, HANNAH R. MORRIS, & PAUL M. WHITMORE
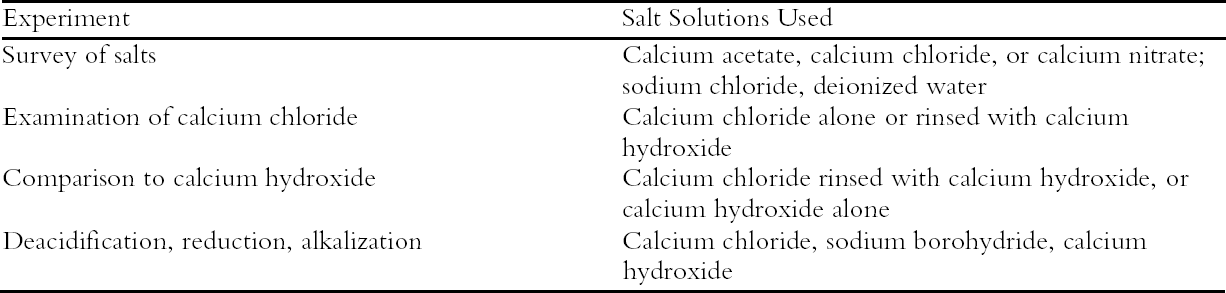
2 EXPERIMENTAL APPROACHThe focus of these experiments was to evaluate the effects of concentrated salt solutions on oxidized cellulose; their effect on other paper components or on ink or other media was not examined. To follow chemical changes in the samples, tests were performed on a pure cotton cellulose filter paper, Whatman no. 42, that was oxidized prior to treatment by exposure to ultraviolet-A lamps (emitted wavelengths 320–400 nm). Exposure times were different for each experiment and ranged from 70 to 168 hours. Some of the exposed sheets were also thermally aged at 90�C and 50% RH prior to treatment. The photo-oxidation and accelerated aging of the filter paper was an attempt to simulate the deteriorated papers encountered by conservators. Chemical tests of the treated sample sheets were performed before and after accelerated thermal aging. The details of these tests are described in appendix 1 and will be briefly summarized here. The viscosity of cellulose solutions in cupriethylenedi-amine was measured to calculate the degree of polymerization (DP) of the cellulose chains. From the D P, the concentration of scissions, or new breaks in the chains, was calculated. Graphs of the degradation of the samples upon aging have been drawn in terms of the scissions, which illustrate more clearly than the DP the progress of the degradative reactions. The concentrations of carbonyl and carboxyl oxidation groups were measured using colorimetric methods: the hydrazine technique for the carbonyl group and the methylene blue method for the carboxyls. The units for the concentrations of oxidation groups and scissions have been standardized to mmol/100 g to allow for easy comparison. As noted in an earlier paper (Whitmore and Bogaard 1994), the changes in oxidation group content can be compared to the scissions produced to discern the dominant degradation pathway. If these numbers are approximately equal, acid-catalyzed hydrolysis is indicated. Significantly higher oxidation changes of at least two to three times more than the scissions indicate an oxidative process. The pH was measured using the standard cold extract method, usually with the modification of soaking in a solution of sodium chloride (Scallan 1990) rather than deionized water. The brightness of the sheets was tracked and is expressed in terms of % reflectance (%R) at 460 nm, which indicates yellowing of the paper by a decrease in reflectance. No other reflectance changes besides the decrease at low wavelengths (i.e., no other color changes besides yellowing) were observed. The concentrated salt solutions were 0.1 M and had a pH that ranged from 6.7 to 7.9; the concentrated solutions of calcium chloride ranged in pH from 7.2 to 7.4. The dilute calcium hydroxide solutions were prepared from 40-fold dilutions of a saturated calcium hydroxide solution, approximately 0.4 mM in concentration, with a pH around 10. For all the treatments, paper was immersed in the designated solution for only about 15-30 minutes; when more than one bath was used, samples were immersed successively without drying. Four sets of experiments were performed on the photo-oxidized filter paper, and they are summarized in table 1. First was a survey of the deacidifying effects of concentrated solutions of four different neutral or slightly alkaline salts (calcium acetate, calcium chloride, calcium nitrate, and sodium chloride) compared to deionized water. The next experiment examined the effects of concentrated calcium chloride solutions, with and without a following rinse of dilute (approx. 0.4 mM) calcium hydroxide. The third experiment employed photo-oxidized sheets that were thermally aged prior to treatment to create more degraded, acidic samples. A comparison was
The last experiment demonstrated a series of treatment steps that included a chemical reduction to suggest a possible way that a paper conservator could integrate the use of concentrated salt solutions into a treatment regimen. To simulate the aged, degraded papers that are difficult to treat, sample sheets were selected that had been aging at room temperature for more than seven years after exposure to near-ultraviolet light. The samples were treated through a series of seven steps: (1) immersion in the concentrated calcium chloride solution; (2) and (3) two successive rinses in an extremely dilute solution of calcium hydroxide (approx. 0.08 mM, a 200-fold dilution of the saturated solution and about five times more dilute than the calcium hydroxide solution used in the previous experiment); (4) immersion in a bath of 0.01 M sodium borohydride to chemically reduce the cellulose; (5) and (6) two more rinses with the 0.08 mM calcium hydroxide solution; and (7) a final soak in 0.4 mM calcium hydroxide solution. The solution of sodium borohydride used here was about 25 times more dilute than the solution used in our previous work (Bogaard and Whitmore 2001); both strengths are within the range known to be used by conservators (Tang 1986). To follow the uptake of calcium by the sample sheets, as well as to ensure that the chloride ions were rinsed out, contents of calcium and chloride ions in the sample sheets were measured using ion-selective electrodes attached to the pH meter. |