ANALYSIS OF LAMINATED DOCUMENTS USING SOLID-PHASE MICROEXTRACTIONMark Ormsby
3 PROCEDUREFive types of SPME fibers (from Supelco) with different polymer coatings were evaluated. Table 2 lists the fibers and their recommended applications. The fibers were initially conditioned by heating in the GC-MS injector at a temperature as recommended by the manufacturer. Analysis was performed on an HP6890N/5973 Inert GC-MS with a 30-meter HP5-MS (5%phenyl)-methylpolysiloxane column (Agilent). A Supelco narrow-bore inlet liner optimized for
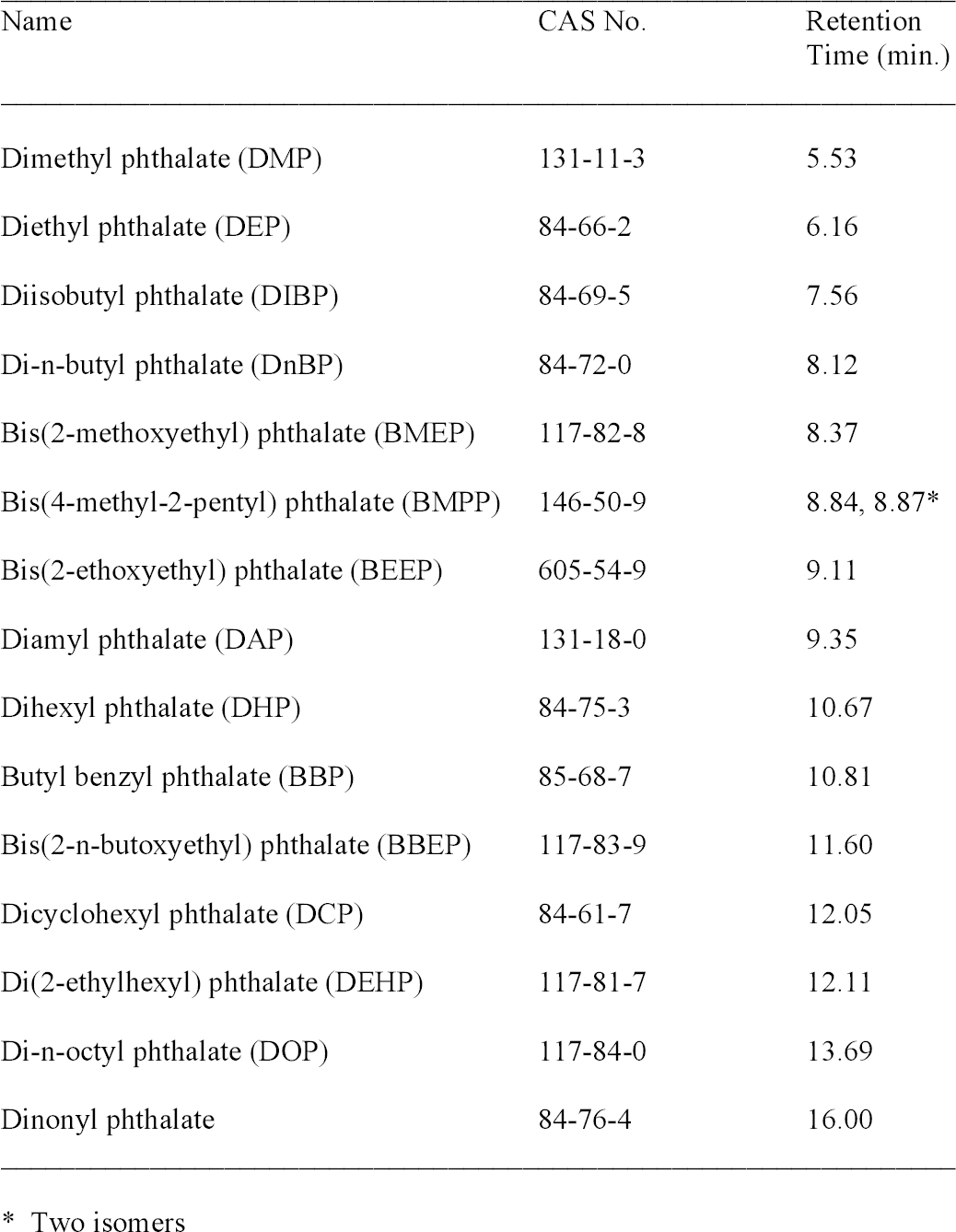
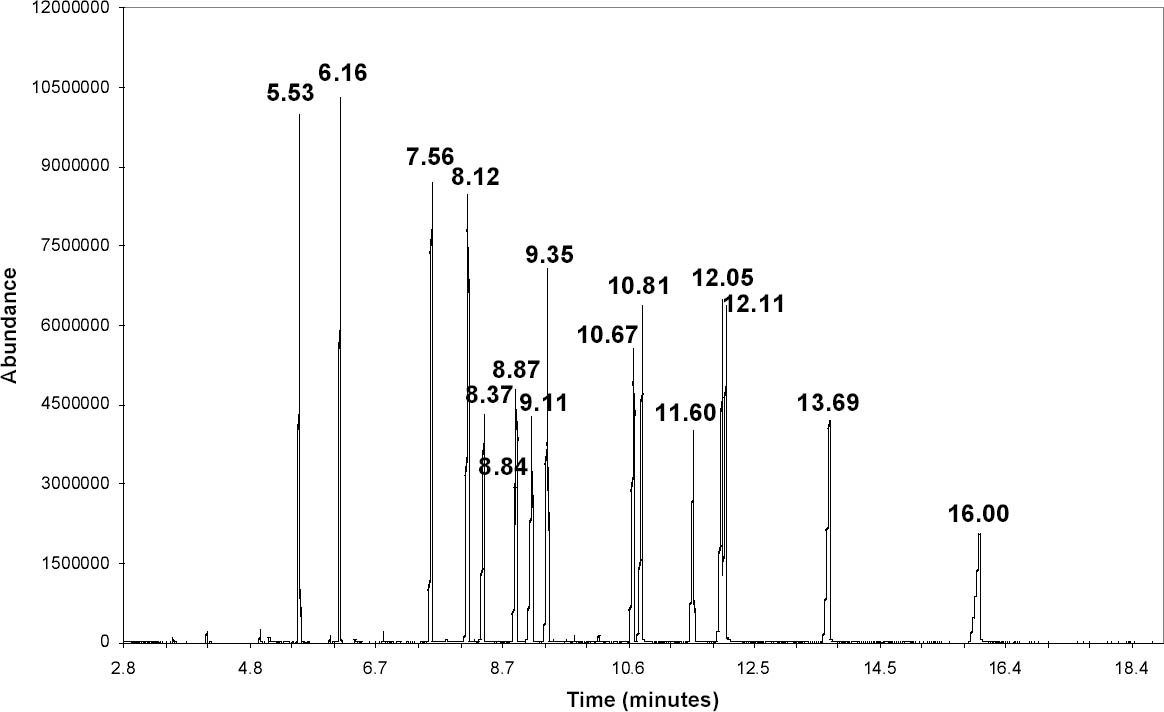
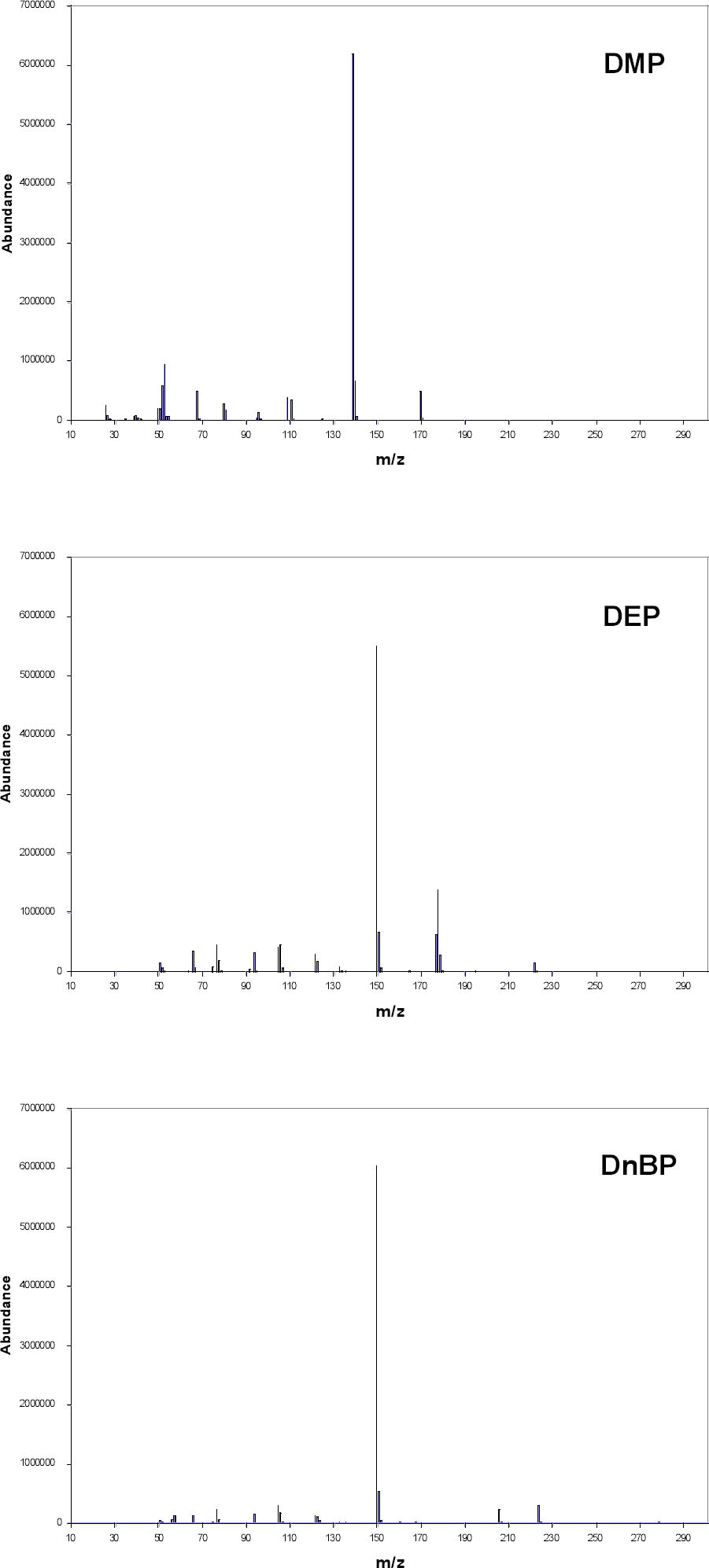
A splitless injection was made with the injector port at 250�C. The carrier gas was helium at 1 ml/minute in the constant flow mode. The oven temperature was initially 50�C. It was heated at 30�C/minute to 210�C and then raised at 10�C/minute to 275�C, where it was held for seven minutes. Mass spectra were collected using electron ionization and a quadrupole mass filter. The MS source, quadrupole, and detector temperatures were 230�, 150�, and 280�C, respectively. A standard spectra autotune using perfluorotributylamine was performed for comparison with the National Institute of Standards and Technology/Environmental Protection Agency/National Institutes of Health (NIST/EPA/NIH) Mass Spectral Library 2002 (Agilent). Spectra were collected in the scan mode over the range 35–400 amu. A standard solution (NSI Solutions) containing 15 phthalates at approximately 1 mcg/ml concentration in hexane was used to optimize the GC-MS settings. To reduce the baseline from the hexane, the lower end of the mass range was raised to 45 (compared to 35 for all other data). Samples were collected using DI-SPME. The total ion chromatogram (TIC) in figure 3 shows that these 15 compounds can be distinguished based on the retention time, the time required for a peak to elute from the column. Retention times were reproducible to approximately � 0.03 minute. An unknown can possibly be identified by determining its retention time (table 3). This set of standards does not include all possible phthalates, however, and it is possible that a different compound might have the same retention time as one of the standards. The identity of a peak can possibly be clarified by examining its electron ionization mass spectrum (fig. 4) and comparing it to the spectral library. Ideally, the mass spectrum serves as a fingerprint for a specific compound, but this is not true in all cases. Di-n-butyl phthalate (DnBP) and some other phthalates have mass spectra with few features besides a large peak at mass-to-charge ratio (m/z) 149. This peak is common to all alkyl phthalates with alkyl
As part of its research projects in the 1950s, NBS prepared experimental CA films made with various combinations of plasticizers and other additives. Samples of these films were taken by cutting a small piece approximately 1 cm2 and placing it in a 10 ml glass vial capped with a polytetrafluoroethylene/silicone septum (Supelco). A SPME fiber was inserted through the septum, exposed to the headspace for 10 minutes, removed, and immediately analyzed. Preliminary testing indicated that the 10-minute exposure provided an adequate signal in a relatively short time. The sensitivity of this method could be improved by using a stir bar or heat (Supelco 1999). This procedure was repeated for five SPME fiber types and various experimental films. To check for carryover of sample on a fiber, a blank run was made between each exposure to a film. To compare the sensitivity of the fibers, the area of the plasticizer peaks was calculated using the ChemStation analysis program. These qualitative tests indicated that the polyacrylate (PA), carbowax-divinylbenzene (CWDVB), and polydimethylsiloxane-divinylbenzene (PDMS-DVB) fibers were suitable for HS-SPME detection of the plasticizers dimethyl phthalate (DMP), diethyl phthalate (DEP), and triacetin as well as phenol, discussed below. These results agree with a study of fiber types used for detecting phthalates in water (Penalver et al. 2000). The PDMS fiber was much less sensitive to phenol. For all four compounds the carboxen-polydimethylsiloxane (CAR-PDMS) fiber had much lower sensitivity. It may be useful, however, for studying the acetic acid
The procedure was modified for documents and objects from which a sample could not be cut and placed in a vial. For HS-SPME, a small beaker or petri dish top was placed on the document, and the fiber was exposed by slipping it under the edge of the glass (fig. 5). When possible, a small drop of ethanol was placed on the document for DI-SPME. TPP was easily detected using this method, although it usually was not found with HS-SPME because of its low volatility. This procedure was assumed not to cause damage to the document because of the minimal exposure to ethanol, but, as a precaution, the drop was placed on the border of laminated documents in an area that contained only the plastic. |