HUNTING FREE AND BOUND CHLORIDE IN THE WROUGHT IRON RIVETS FROM THE AMERICAN CIVIL WAR SUBMARINE H. L. HUNLEYN�STOR G. GONZ�LEZ, PHILIPPE DE VIVI�S, MICHAEL J. DREWS, & PAUL MARDIKIAN
3 RESULTS AND DISCUSSIONAs part of the process of evaluating the current state of the cast and wrought iron of the hull and its components and prior to beginning the extraction experiments, some preliminary analysis was undertaken on the rivets that were removed as part of the excavation of the interior of the Hunley. For the excavation of the submarine's interior, four hull plates were removed. To remove the hull plates, the rivets holding them in place, approximately 100 per plate, were removed from the outside in. Each rivet was bored and drilled for removal, identified and labeled and then stored in either tap water or a 2 wt% caustic soda solution made from tap water. In addition, the rivet shavings from the boring and drilling were also stored, either dry or in tap water or 2 wt% caustic solution made from tap water. It should be noted that the chloride content of the tap water was monitored over the period covered in this investigation, and it varied from 17 to 30 ppm Cl-1.
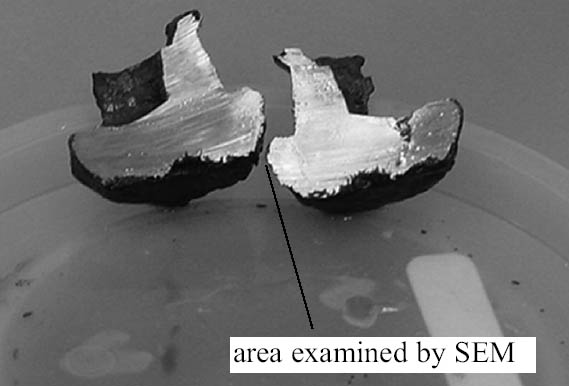
3.1 PRELIMINARY RIVET ANALYSISTo analyze the extent of corrosion prior to the extraction experiments, a special holder was designed and fabricated to hold the rivets to be evaluated for vertical sectioning using the diamond saw. In figures 2 and 3, images of rivet HL-1807 (stored in tap water) before and after cutting illustrate the overall condition of the surface and of the interior. As shown in figure 3, all the apparent corrosion appears to be confined primarily to the surface of the artifact. As part of this experiment, water displacement was used to estimate the density of the rivet. The estimated density was 5.2 g/cm3, which was approximately 70% of the density of a forged steel test specimen used for comparison. These density results were considered consistent with the visual appearance of
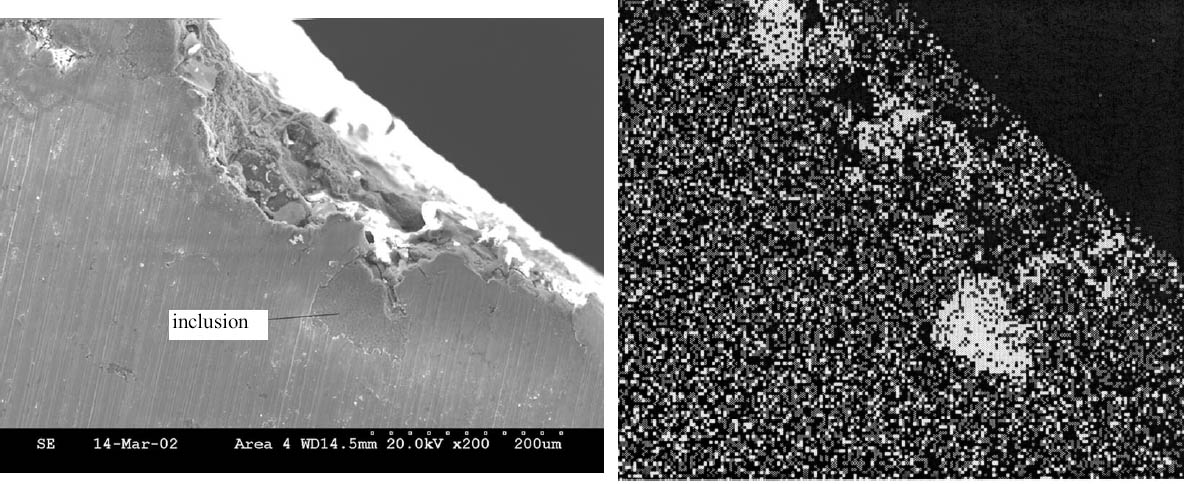
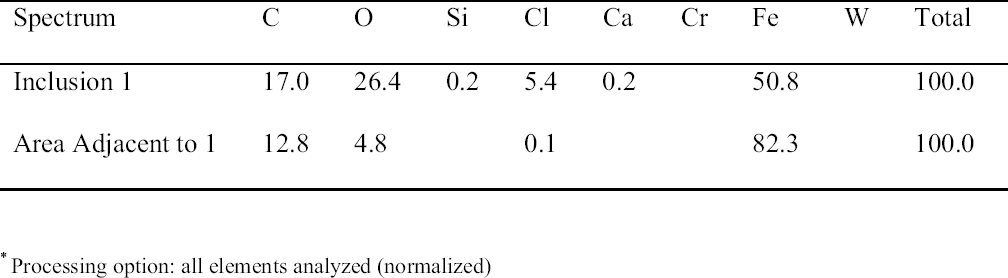
Rivet areas that were examined during these experiments included a remnant of the artifact that was on the outside of the hull where extensive corrosion was observed. The corrosion pattern observed in this area was consistent with patterns found in other areas of the rivet and was characterized by an irregular growth front with channels leading well below the average depth of the corrosion. Whether this pattern represented movement along grain boundaries has not yet been established. Somewhat surprisingly, only a few areas of high chlorine concentration were detected by EDX in these corroded regions. On the other hand, an area (identified in fig. 3) on the head of the rivet inside of the hull, which included an internal inclusion, displayed much higher levels of Cl. A micrograph of this region along with a Cl map is shown in figure 4, and the qualitative analysis results are summarized in table 2. In addition to the high levels of chlorine in this inclusion, as shown in figure 4, high levels were also observed along most of the corrosion front in this area. The corrosion products in the shavings were assumed to be similar to those on the rivet exterior because they were obtained from adjacent areas. It was also assumed that because of their relatively small size and high surface-to-volume ratios, as compared to an intact rivet, extraction from the rivet shavings would be more efficient. Finally, because of their different storage conditions, this set of samples could also represent what might happen if a recovered artifact were allowed to simply dry out or were stored in plain tap water instead of immersed in a caustic solution shortly after recovery. 3.2 DIGESTION OF SHAVINGS FROM SETS A, B, AND CThe principal samples used in this part of the study were the metal shavings generated when the rivets were removed by drilling from the hull plates of the H. L. Hunley prior to their removal for the excavation of the interior of the submarine. After their removal in February 2001, the rivets from one of the hull plates were stored in tap water and those from the other in 2 wt% NaOH solutions made up
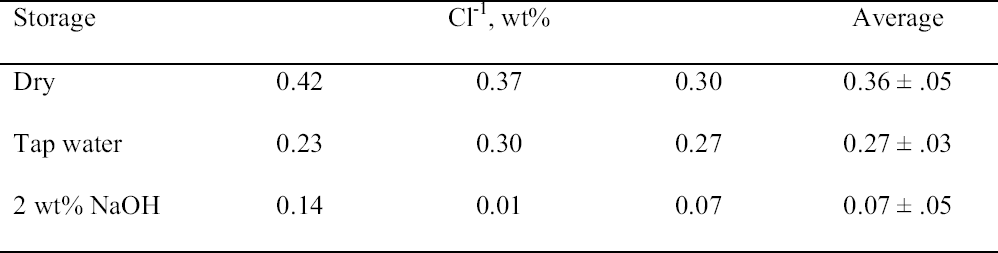
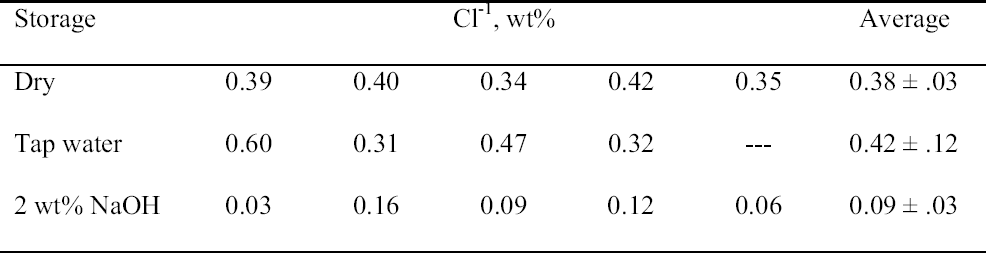
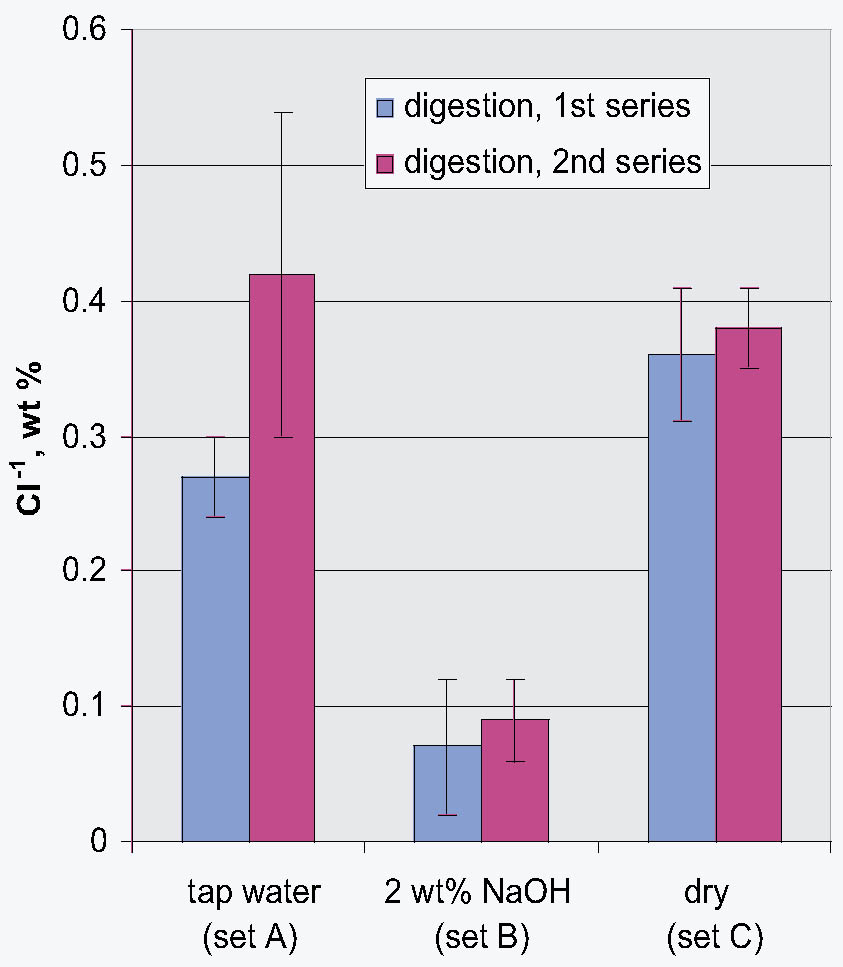
That the storage conditions had a significant effect on the physical appearance of the rivet shavings was immediately obvious, as shown by the photographs of representative samples from each set shown in figure 5. The shavings stored dry (set C) were reddish brown and contained a significant amount of powdery residue. The shavings stored in tap water (set A) were darker in color, and the storage solution contained a significant amount of suspended colloidal material. The shavings stored in 2 wt% NaOH (set B) were very dark, almost black, and the storage solution contained almost no suspended material and was very clear. Because of the heterogeneous nature of these shavings and their corrosion products, as illustrated by the samples shown in figure 5, for each of the digestion or extraction experiments special care was taken to select representative samples. The approach chosen to ensure representative sampling was to start with a much larger sample, distribute it evenly on a surface, and then randomly select subsamples of the appropriate size. Prior to carrying out the extraction experiments, the total weight percent Cl-1 content of each set of rivet shavings was determined by digestion. In the first series of digestion experiments, triplicate samples from each set were analyzed. The results are summarized in table 3. As clearly shown by these data, the storage conditions affected the Cl-1 content of the rivet shavings as well as their physical appearance. As expected, storage in 2 wt% NaOH (set B) had the most dramatic effect on both the physical appearance and Cl-1 content of the shavings, with a Cl-1 content at least 75% less than that measured for the other two conditions. To evaluate the reproducibility of the digestion and sampling procedures, as well as to obtain more statistical data to allow for a comparison of the dry- and tap-water–stored shavings, a second series of digestions with five replicates each was undertaken. The results from these experiments are summarized in table 4, and the averages and standard deviations from the two data sets are compared in figure 6. When the data in tables 3 and 4 are compared, it is obvious that the two wet samples (sets A and B) are the most difficult to sample reproducibly and generate
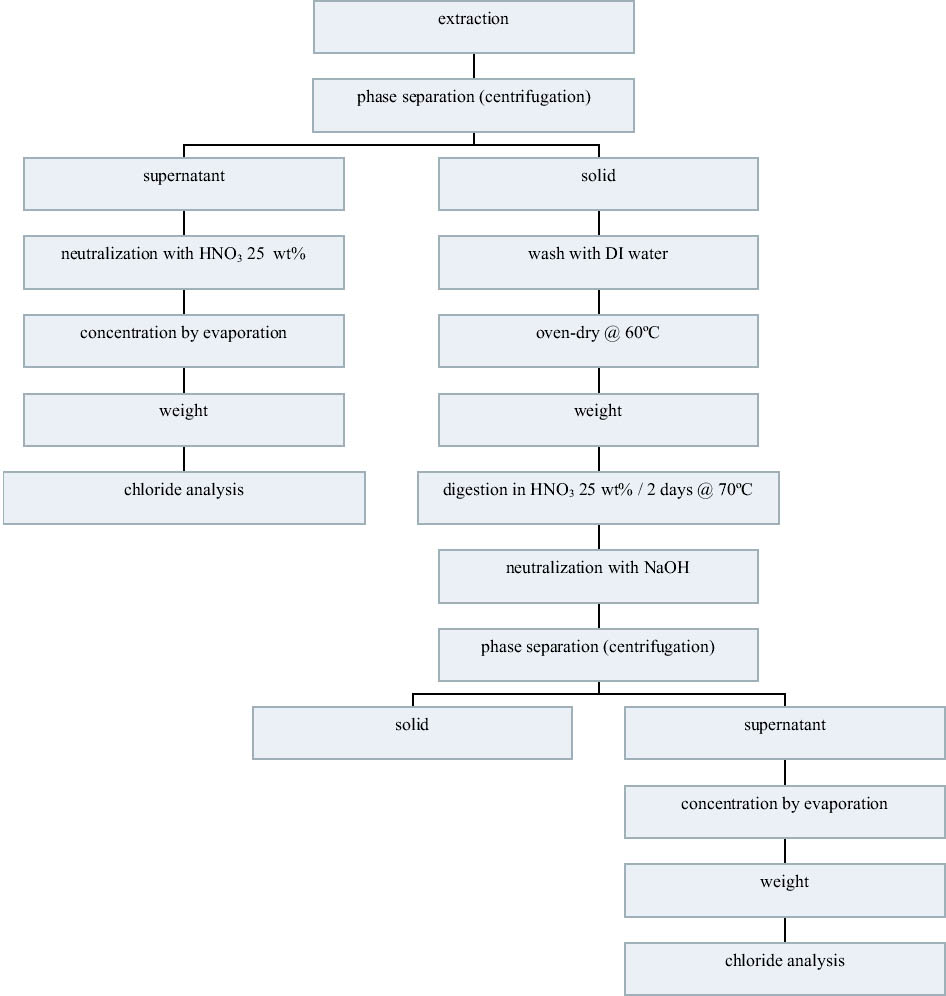
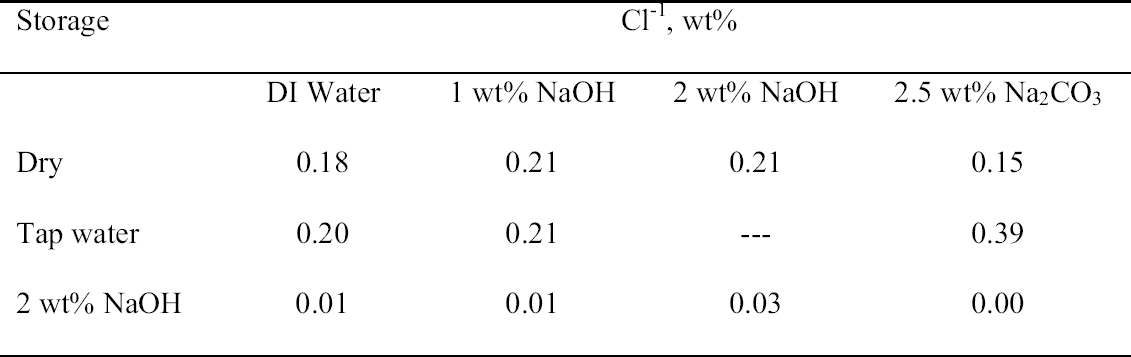
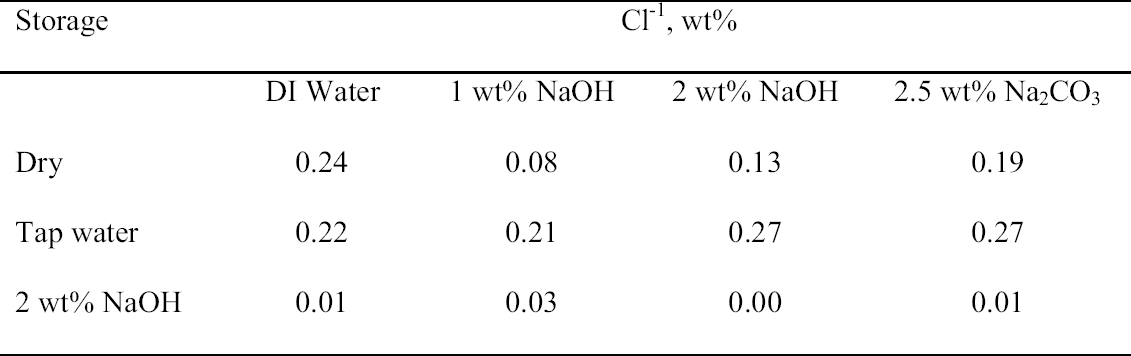
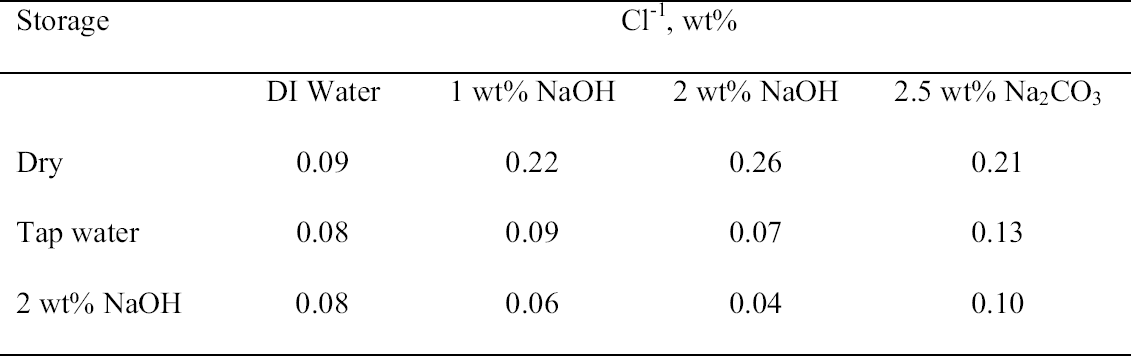
3.3 72-HOUR EXTRACTION OF SHAVINGS FROM SETS A, B, AND CIn the next sequence of experiments, rivet shavings selected and prepared as for the digestions were extracted using a variety of solutions in an attempt to characterize how easily removable the Cl-1 content was in each set. For the initial extraction experiments, an extraction time of 72 hours and DI water, 2.5 wt% Na2CO3 and 1 or 2 wt% NaOH in DI water solutions, were employed. The alkaline solutions were selected based on their frequent utilization in the conservation literature for the removal of Cl-1 from iron artifacts. The DI water was used for comparative purposes and to represent an extraction solution that would be expected to remove only relatively free Cl-1 without significant counterion replacement. The data for wt% Cl-1 extracted from the rivet shavings after 72 hours are summarized in table 5. When the results summarized in table 5 are compared to the digestion data, it is clear that except for the extraction of the rivet shavings stored in tap water with the 2.5 wt% Na2CO3, only ~ 50% of the Cl-1 was removed in these experiments. On the other hand, the Na2CO3 result may simply reflect the extraction of a high Cl-1 tap water sample, as the data from the set A digestion samples ranged from a low of 0.23 to a high of 0.60 wt% Cl-1. As with the digestion results, the extraction results are consistent with the conclusion that the Cl-1 content of the rivet shavings stored dry and stored in tap water (sets C and A) was the same and significantly higher than in the shavings stored in NaOH (set B). Surprisingly, there appeared to be no obvious difference in the extraction efficiencies of any of the solutions used. 3.4 14-DAY EXTRACTION FOLLOWED BY DIGESTION OF SHAVINGS FROM SETS A, B, AND CConsequently, a second set of extraction experiments using a time of 14 days, with and without stirring the samples, was performed. Since no difference was apparent in the extraction results for the samples that were agitated and those that were not, these data were combined, and these results are summarized in table 6. A comparison of the data in tables 5 and 6 clearly shows that, overall, the extraction efficiencies at 14 days were very similar to those observed at 72 hours. In fact, if the average Cl-1 extracted from the shavings stored dry and in tap water (sets C and A) is calculated, at 72 hours it was 0.22 � 0.07, and at 14 days it was 0.20 � 0.06 wt%, which are statistically the same. Similarly, for the shavings stored in NaOH (set B), there was no statistical difference between the two extraction times. It is also clear that even though the Cl-1 content of the rivet shavings stored in NaOH was significantly lower than in the other two samples, very little of this remaining chloride appears to be readily extractable. To compare the extraction data and the digestion results more directly, the solids remaining after the 14-day extraction were then digested, and these results are summarized in table 7. Perhaps the most interesting observation that can be made regarding the data in table 7 is that for the shavings stored in the two solutions, the wt% Cl-1 remaining in the shavings after extraction appears to be statistically almost the same: 0.09 � 0.02 (set A, tap water) and 0.07 � 0.02 (set B, NaOH) and significantly less than in those stored dry, 0.20 � 0.06 wt% (set C). To view these data from a different perspective, the data from tables 6 and 7 as well as the shaving digestion data from the three sets of shavings are compared in graphic format in figure 7. Presented in this manner, these results clearly show the effects of the changes in the rivet storage conditions on the total amount of readily extractable Cl-1. In addition, as might be expected, the dry shavings from set C appeared to contain the least amount of readily extractable Cl-1, and those stored in tap water from set A the most. Whether any additional Cl-1 could be removed by extracting for a longer period of time from any of these samples is uncertain at this time. Also, as is illustrated in figure 7, even after storage for a period of almost two years in 2 wt% caustic solution, a significant concentration (greater than 500 ppm) of very difficult to remove Cl-1 remained in the rivet shavings from set B. Whether this concentration represents Cl-1 that is chemically bound to a corrosion product, that is trapped as an inclusion in a corrosion product such as akagan�ite, or that is simply deep within the corrosion pore structure has yet to be determined.
|