HUNTING FREE AND BOUND CHLORIDE IN THE WROUGHT IRON RIVETS FROM THE AMERICAN CIVIL WAR SUBMARINE H. L. HUNLEYN�STOR G. GONZ�LEZ, PHILIPPE DE VIVI�S, MICHAEL J. DREWS, & PAUL MARDIKIAN
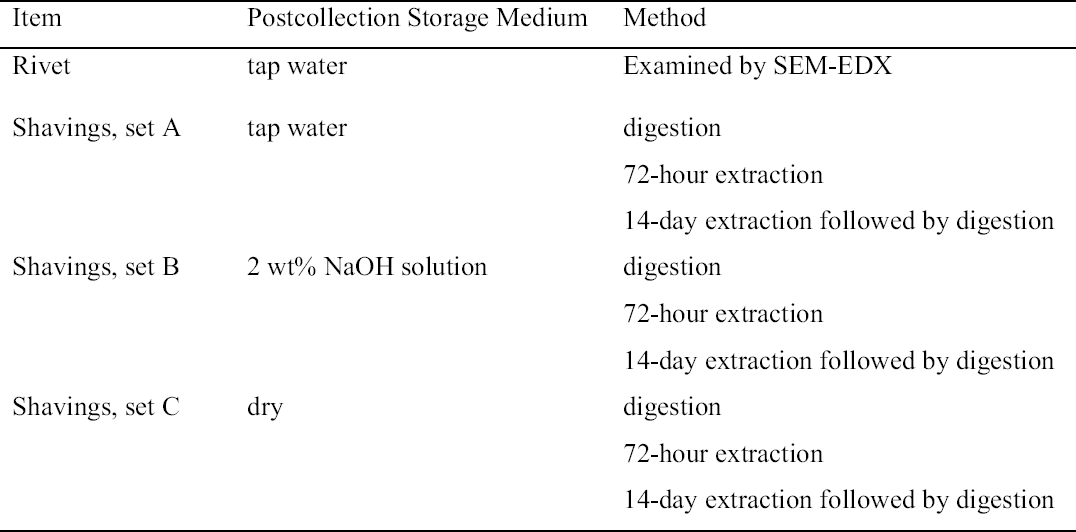
2 EXPERIMENTAL2.1 SAMPLE SETSThe samples used in this study were the remnants of the rivets and the metal shavings generated when the rivets were drilled in order to remove them from the hull plates of the H. L. Hunley prior to the excavation of the interior of the submarine. After their removal in February 2001 and until they were employed in this investigation, the rivets from one of the hull plates were stored in tap water and those from the other in 2 wt% sodium hydroxide (NaOH) solutions made up with tap water. In this initial investigation, only rivets stored in tap water were used. The respective rivet shavings were also stored in tap water (set A), and in 2 wt% NaOH (set B) solutions also made up with tap water. When another plate was removed in January 2002, these rivet shavings were allowed to air-dry and stored dry (set C). All the samples and the experiments performed on the three sets of shavings in this investigation are summarized in table 1. The chloride content of the tap water used to store the samples was analyzed periodically. 2.2 RIVET PREPARATION AND ANALYSISTwo rivets that had been stored in tap water were cut into two sections using a Buehler, 5 in. diameter � 0.015 in., diamond wafering blade, cooled with a spray of deionized water, in a Buehler Isomet 2000. After cutting, the rivet halves were mounted on aluminum stubs with carbon tape, and the cut surfaces were examined by scanning electron microscopy (SEM) and energy dispersive x-ray spectroscopy (EDX) using a Hitachi S3500N scanning electron microscope operated at an accelerating voltage of 20 KeV and equipped with an Oxford INCA 400 energy dispersive x-ray analyzer. 2.3 BULK SHAVING SET SAMPLE PREPARATION AND EXTRACTIONFor samples stored in solution, approximately 15 g of the wet shavings were weighed and then dried in an isothermal oven (Fisher Isotemp) at 60oC, cooled, and then reweighed. No quantitative chloride analysis was conducted on the storage solutions because the initial sample volume was not recorded and evaporation during storage was not monitored.
For each individual extraction sample, a carefully selected representative sample of approximately 2.5 g was weighed using a 0.001 g analytical balance (Denver Instruments Model TR-403) into preweighed, 250 ml, high-density polyethylene specimen vials containing 80 ml of either 17 MegOhm deionized (DI) water, or 2.5 wt% sodium carbonate (Na2CO3) (Fisher Certified ACS Reagent Grade, anhydrous) in DI water, or 1 or 2 w% NaOH (Fisher, 50 wt% for Kjeldahl determinations) in DI water. Duplicate samples were extracted for 72 hours or 14 days at room temperature with and without stirring. After extraction, the solid and liquid phases were separated by centrifuging at a relative centrifugal force (RCF) of 1950 (Thermo IEC, Centra Cl-2) for five minutes. The solid phase was then washed with DI water, and the washing solution was added to the supernatant. The washed solid was dried overnight in the isothermal oven at 60�C, cooled to room temperature, and reweighed prior to digestion to determine the Cl-1 remaining after extraction. The supernatant was neutralized with 25 wt% in DI water nitric acid (HNO3) (90 wt%, Fisher Certified ACS Reagent Grade) to a final pH of 6–7.5. The pH was measured using a BDH Gelplas, double junction pH electrode attached to an Orion Model 520 pH meter. After neutralization the solution was concentrated to a final volume of ~ 40 ml on a hot plate at a temperature below the boiling point to avoid the uncontrollable loss of liquid as a result of bubbling. Since all the chloride analysis was done on a w/w basis in terms of parts per million (ppm) Cl-1, the actual final sample volume was not critical, only the final sample mass. 2.4 DIGESTION OF THE SHAVING SET SAMPLESFor each digestion, approximately 0.7 g of dried solid, either before or after extraction, were weighed to �0.001 g into a preweighed 125 ml Erlenmeyer flask, and 25 wt% in DI water HNO3 in a ratio of ~ 20 times the weight of the sample was added. The samples were digested for 48 hours on a hot plate, then cooled to room temperature and neutralized initially with 50 wt% NaOH and then to a final pH of 6–7.5 using 1 wt% NaOH. The supernatant was separated from the solid by centrifugation and then concentrated on a hot plate. The Cl-1 ion concentrations were measured using Hach Quantab, low range, 30–600 ppm, titrator strips as well as by potentiometric titration. For the potentiometric titration, a Thermo Orion 960 autochemistry system equipped with a Thermo Orion Ionplus, double junction Cl-1 ion selective electrode was used. The titrant used was, depending on the Cl-1 concentration, 0.1N, 0.01N, or 0.001N silver nitrate (AgNO3) (made by diluting Fisher Certified 1N standard AgNO3 solution, 1:10, 1:100, 1:1000). The end point was determined using the second derivative method, and the method was calibrated using standard Cl-1 solutions. The limit of detection using this method as it was employed on the samples analyzed in this investigation was experimentally determined to be less than 100 ppm, which corresponds to a value of less than 0.01 wt%. A flowchart of the overall analysis scheme is shown in figure 1. |