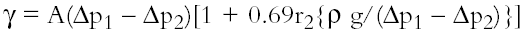FINDING SUBSTITUTE SURFACTANTS FOR SYNPERONIC NJOHN A. FIELDS, ANDREW WINGHAM, FRANCES HARTOG, & VINCENT DANIELS
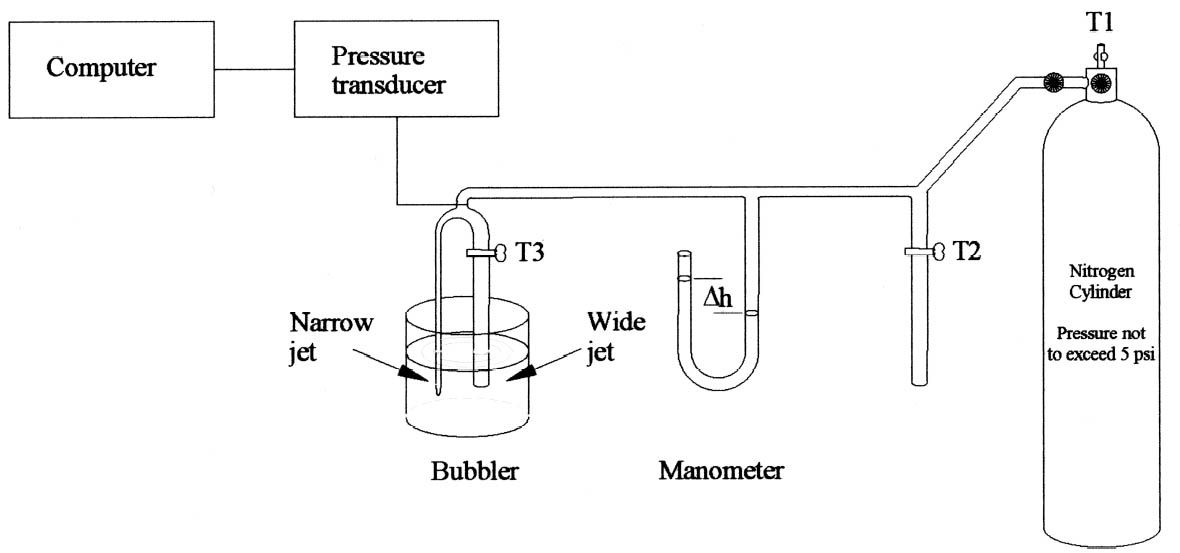
3 SUGDEN'S BUBBLE METHODAs air bubbles leave a capillary tube immersed in a liquid, the pressure of the air in the tube varies as the bubble is formed and then leaves the capillary. The pressure change (Δp) required is dependent on the depth to which the tube is immersed into a liquid (w), the density (ρ) and surface tension (γ) of that liquid, and the radius of the capillary bore (r). Sugden's apparatus (fig. 2) employs two capillaries with one narrow-bore capillary and another wide one, in this case 1 and 5 mm. In this apparatus, the variation in pressure of nitrogen bubbles as they were formed and left the capillary was recorded graphically by a transducer connected to a computer for recording results. The pressure transducer consisted of a silicon diaphragm. The disc distorts when pressure is applied, in turn altering the electrical resistance of the silicon in an anisotropic way, the resulting resistance difference reflecting the pressure difference.
The apparatus is calibrated with toluene to obtain the apparatus constant (A). For each surfactant, the pressure change during bubbling in a range of solutions from 0.005% to 0.5% w/v was recorded separately for the narrow and wide capillaries (Δp1 and Δp2), where r2 is the radius of the wide jet. The values were introduced to Sugden's equation, and the surface tension of each solution calculated (g is the acceleration due to gravity) (Sugden 1922, 1924).
A graph of % w/v concentration vs. surface tension (γ) yields two straight lines with an intercept at the CMC (fig.3). The CMC for each of the surfactants was calculated in this manner at room temperature (20�C). The resulting CMC values can be seen in table 1. |