FINDING SUBSTITUTE SURFACTANTS FOR SYNPERONIC NJOHN A. FIELDS, ANDREW WINGHAM, FRANCES HARTOG, & VINCENT DANIELS
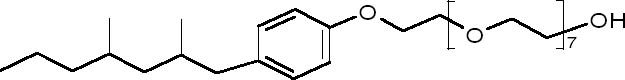
1 INTRODUCTIONSynperonic N, previously known as Lissapol, has been widely used in conservation since its recommendation in Plenderleith's 1956 book on the conservation of antiquities and works of art (Plenderleith 1956). Nonionic surfactants are generally low foaming and unreactive to metal ions. Anionic surfactants may form insoluble compounds with multivalent ions such as calcium and magnesium. Hence the use of anionic surfactants in hard water or, for example, with metals, calcareous stones, and calciumor magnesiumcontaining fibers such as paper, produce problematic insoluble deposits. In such cases, a nonionic surfactant may be a better choice. Synperonic N is a 27% (v/v) solution of the nonylphenol ethoxylate (fig. 1) nonionic surfactant Synperonic NP8, originally manufactured by Imperial Chemical Industries (ICI) and, until 1999, by its Uniqema subdivision. Degradation of nonylphenol ethoxylates occurs by a progressive shortening of the polyoxyethylene hydrophilic chain, making the compound progressively less soluble and increasingly associative to other suspended solids. The shortening of the chain also makes further biodegradation more difficult, and therefore—although nonylphenol ethoxylates are ultimately biodegradable, i.e., they conform to EU directive 82/242/EEC—their degradation products are considered more harmful than their precursor. In vitro studies showed that nonylphenol has an estragenic activity three to six orders of magnitude less potent than estradiol (Jobling and Sumpter 1993; Jobling et al. 1996).
In 1974, the Paris Commission (PARCOM) was set up as the European Commission's (EC) body responsible for the prevention of marine pollution from land-based sources, and in 1992, EU PARCOM recommendation 92/8 concluded that high-tonnage uses of nonylphenol ethoxylates result in a large degree of aquatic exposure to potentially toxic degradation products. The recommendation followed that nonylphenol ethoxylates be phased out for domestic purposes by 1995 and for industrial use by 2000. ICI no longer sells these products within the EU. Although a range of Synperonic surfactants still exists, in these a large alkyl group replaces the hydrophobic nonylphenol group of the Synperonic N. The fact that Synperonic N is no longer available has dealt a major blow to the conservation community in Europe, where it was the surfactant of choice, as it was the only surfactant recommended by a scientist (Plenderleith 1956) and has proved highly efficient on cotton and wool. In the few comparison investigations with an alternative nonionic detergent used in Europe (Gentle and Muller 1995; Lewis and Eastop 2001), the results were inconclusive or served to underline the cleaning power of Synperonic N. The need to replace Synperonic N is immediate due to the phasing out of its use in 2000. Its replacement Definitions of technical terms can be found in Appendix 1. |