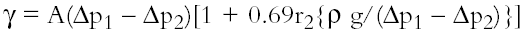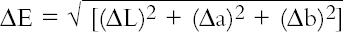FINDING SUBSTITUTE SURFACTANTS FOR SYNPERONIC NJOHN A. FIELDS, ANDREW WINGHAM, FRANCES HARTOG, & VINCENT DANIELS
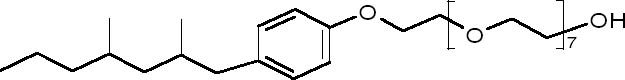
ABSTRACT—Environmental concerns have led to a cessation in Europe of the manufacture of the popular nonionic surfactant Synperonic N. This suspension has dealt a major blow to the conservation community, especially in the United Kingdom, where it is the primary surfactant used. A substitute was needed, and so from an initial list of 24 possible replacements, 11 were selected for testing. The critical micelle concentration for each of the 11 surfactants was determined experimentally, as it was not available from every manufacturer. By testing washing efficiency through observation of color change before and after washing, and also recording the pH and conductivity of the wash liquor, the 11 surfactants were reduced to 5. Further testing of the remaining 5 surfactants involved accelerated aging of washed textiles and measurement of the resulting color, pH, and tensile strength. Although Dehypon LS45 yielded the best cleaning properties on wool, any of the other 4 remaining surfactants (two nonionic surfactants, Synperonic 91/6 and Imbentin C135/070; and two anionic surfactants, Hostapon T and Orvus WA paste) could be used as replacements. TITRE—� la recherche de d�tergents tensio-actifs pour remplacer le Synperonic N. R�SUM�—Des pr�occupations environnementales ont entra�n� l'arr�t de la fabrication en Europe de l'agent de surface non ionique populaire Synperonic N. Cette suspension s'est fait sentir au sein de la communaut� des conservateurs-restaurateurs, particuli�rement au Royaume-Uni, o� le Synperonic N est le d�tergent tensio-actif le plus couramment utilis�. Un produit de remplacement �tait donc n�cessaire et ainsi, � partir d'une premi�re liste de 24 remplacements possibles, 11 produits ont �t� choisis pour des tests. La concentration micellaire critique pour chacun des 11 d�tergents tensio-actifs a �t� d�termin�e exp�rimentalement, car cette information n'�tait pas fournie par tous les fabricants. En examinant l'efficacit� du d�tergent par l'observation des changements de couleur avant et apr�s le lavage, ainsi qu'en mesurant le pH et la conductivit� de l'eau de lavage, le nombre de produits a �t� r�duit � 5. Des tests suppl�mentaires sur ces 5 d�tergents ont consist� au vieillissement acc�l�r� des textiles lav�s et l'effet de ce vieillissement sur les couleurs, le pH, et la force de traction. Bien que le Dehypon LS45 ait d�montr� les meilleures propri�t�s de nettoyage sur des laines, les 4 autres d�tergents tensio-actifs (deux agents de surface non ioniques, le Synperonic 91/6 et l'Imbentin C135/070, ainsi que deux agents de surface anioniques, l'Hostapon T et l'Orvus WA Paste) pourraient �tre aussi bien employ�s comme remplacements. TITULO—B�squeda de agentes tensoactivos substitutos para Synperonic N. RESUMEN— Preocupaciones ambientales han conducido a la suspensi�n de la manufactura del popular agente tensoactivo no polar Synperonic N en Europa. Esta suspensi�n ha sido un gran golpe para la comunidad de conservadores, especialmente en el Reino Unido donde es el principal agente tensoactivo utilizado. Se necesitaba un substituto por lo que de una lista inicial de 24 posibles substitutos, se seleccionaron 11 para ser evaluados. Se determino experimentalmente la concentraci�n micelar critica para cada uno de los 11 agentes tensoactivos ya que no fue proporcionada por todos los productores. Evaluando la eficiencia de lavado a trav�s de observaciones de cambio de color antes y despu�s del lavado as� como registrando el pH y conductividad de las aguas de lavado, los 11 agentes tensoactivos fueron reducidos a 5. Evaluaciones m�s exhaustivas de los 5 agentes tesnsoactivos seleccionados incluyeron envejecimiento acelerado de los textiles lavados y mediciones los resultados en el color, pH y resistencia a la tensi�n. Aunque Dehyphon LS45 present� las mejores propiedades de limpieza para lana, cualquiera de los otros cuatro agentes tensoactivos restantes (2 no-ionicos, Synperonic 91/6 e Imbentin C135/070, y dos i�nicos, Hostapon T y pastaOrvus WA) pueden ser usados como substitutos. T�TULO—Buscando substitutos tenso-ativos para Synperonic N. RESUMO—Preocupa��es com o meio ambiente ocasionaram o fim da fabrica��o, na 1 INTRODUCTIONSynperonic N, previously known as Lissapol, has been widely used in conservation since its recommendation in Plenderleith's 1956 book on the conservation of antiquities and works of art (Plenderleith 1956). Nonionic surfactants are generally low foaming and unreactive to metal ions. Anionic surfactants may form insoluble compounds with multivalent ions such as calcium and magnesium. Hence the use of anionic surfactants in hard water or, for example, with metals, calcareous stones, and calciumor magnesiumcontaining fibers such as paper, produce problematic insoluble deposits. In such cases, a nonionic surfactant may be a better choice. Synperonic N is a 27% (v/v) solution of the nonylphenol ethoxylate (fig. 1) nonionic surfactant Synperonic NP8, originally manufactured by Imperial Chemical Industries (ICI) and, until 1999, by its Uniqema subdivision. Degradation of nonylphenol ethoxylates occurs by a progressive shortening of the polyoxyethylene hydrophilic chain, making the compound progressively less soluble and increasingly associative to other suspended solids. The shortening of the chain also makes further biodegradation more difficult, and therefore—although nonylphenol ethoxylates are ultimately biodegradable, i.e., they conform to EU directive 82/242/EEC—their degradation products are considered more harmful than their precursor. In vitro studies showed that nonylphenol has an estragenic activity three to six orders of magnitude less potent than estradiol (Jobling and Sumpter 1993; Jobling et al. 1996).
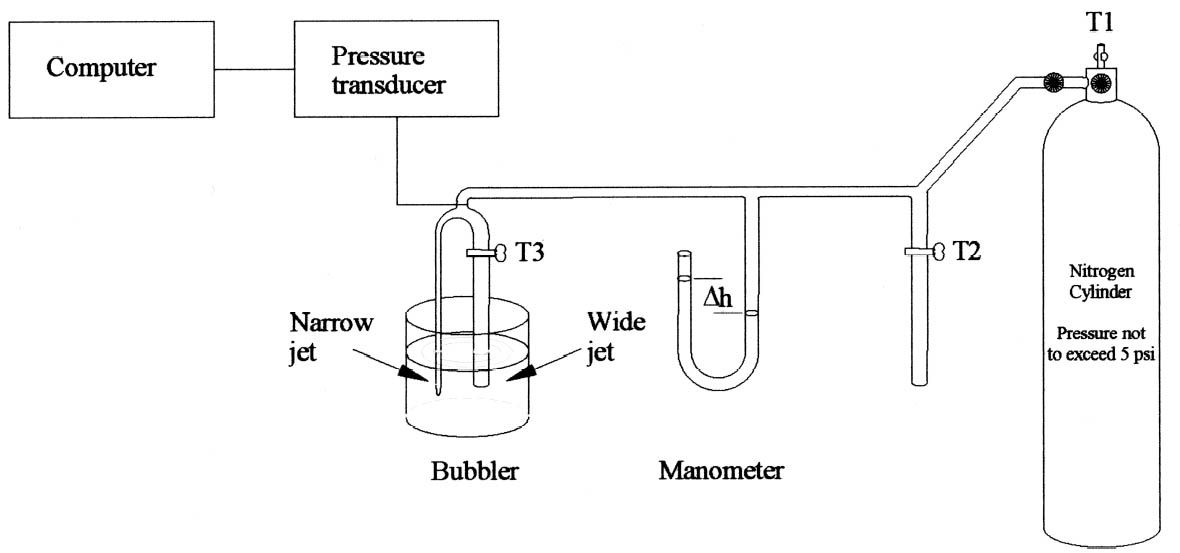
In 1974, the Paris Commission (PARCOM) was set up as the European Commission's (EC) body responsible for the prevention of marine pollution from land-based sources, and in 1992, EU PARCOM recommendation 92/8 concluded that high-tonnage uses of nonylphenol ethoxylates result in a large degree of aquatic exposure to potentially toxic degradation products. The recommendation followed that nonylphenol ethoxylates be phased out for domestic purposes by 1995 and for industrial use by 2000. ICI no longer sells these products within the EU. Although a range of Synperonic surfactants still exists, in these a large alkyl group replaces the hydrophobic nonylphenol group of the Synperonic N. The fact that Synperonic N is no longer available has dealt a major blow to the conservation community in Europe, where it was the surfactant of choice, as it was the only surfactant recommended by a scientist (Plenderleith 1956) and has proved highly efficient on cotton and wool. In the few comparison investigations with an alternative nonionic detergent used in Europe (Gentle and Muller 1995; Lewis and Eastop 2001), the results were inconclusive or served to underline the cleaning power of Synperonic N. The need to replace Synperonic N is immediate due to the phasing out of its use in 2000. Its replacement Definitions of technical terms can be found in Appendix 1. 2 MEASUREMENT OF CRITICAL MICELLE CONCENTRATIONSurfactants are at their most effective at or above their critical micelle concentration (CMC). As the micelles solubilize the soil, the concentration of available micelles is reduced and the surfactant becomes less effective. Therefore, it was decided that the surfactant solutions should be used in concentrations that were several times their CMC. This technique allows for some of the surfactant micelles to be used up during the washing process without reducing the cleaning efficiency. In textile conservation, surfactants are used at 2 to 10 times the CMC, with the higher values saved for heavily soiled textiles. For the purpose of this experiment, the surfactants were tested in a solution at five times their CMC. Most of the manufacturers were unable to supply CMC values, and so the CMC had to be determined experimentally. The technique used for the determination of the CMC was Sugden's bubble method (Sugden 1922, 1924). 3 SUGDEN'S BUBBLE METHODAs air bubbles leave a capillary tube immersed in a liquid, the pressure of the air in the tube varies as the bubble is formed and then leaves the capillary. The pressure change (Δp) required is dependent on the depth to which the tube is immersed into a liquid (w), the density (ρ) and surface tension (γ) of that liquid, and the radius of the capillary bore (r). Sugden's apparatus (fig. 2) employs two capillaries with one narrow-bore capillary and another wide one, in this case 1 and 5 mm. In this apparatus, the variation in pressure of nitrogen bubbles as they were formed and left the capillary was recorded graphically by a transducer connected to a computer for recording results. The pressure transducer consisted of a silicon diaphragm. The disc distorts when pressure is applied, in turn altering the electrical resistance of the silicon in an anisotropic way, the resulting resistance difference reflecting the pressure difference.
The apparatus is calibrated with toluene to obtain the apparatus constant (A). For each surfactant, the pressure change during bubbling in a range of solutions from 0.005% to 0.5% w/v was recorded separately for the narrow and wide capillaries (Δp1 and Δp2), where r2 is the radius of the wide jet. The values were introduced to Sugden's equation, and the surface tension of each solution calculated (g is the acceleration due to gravity) (Sugden 1922, 1924).
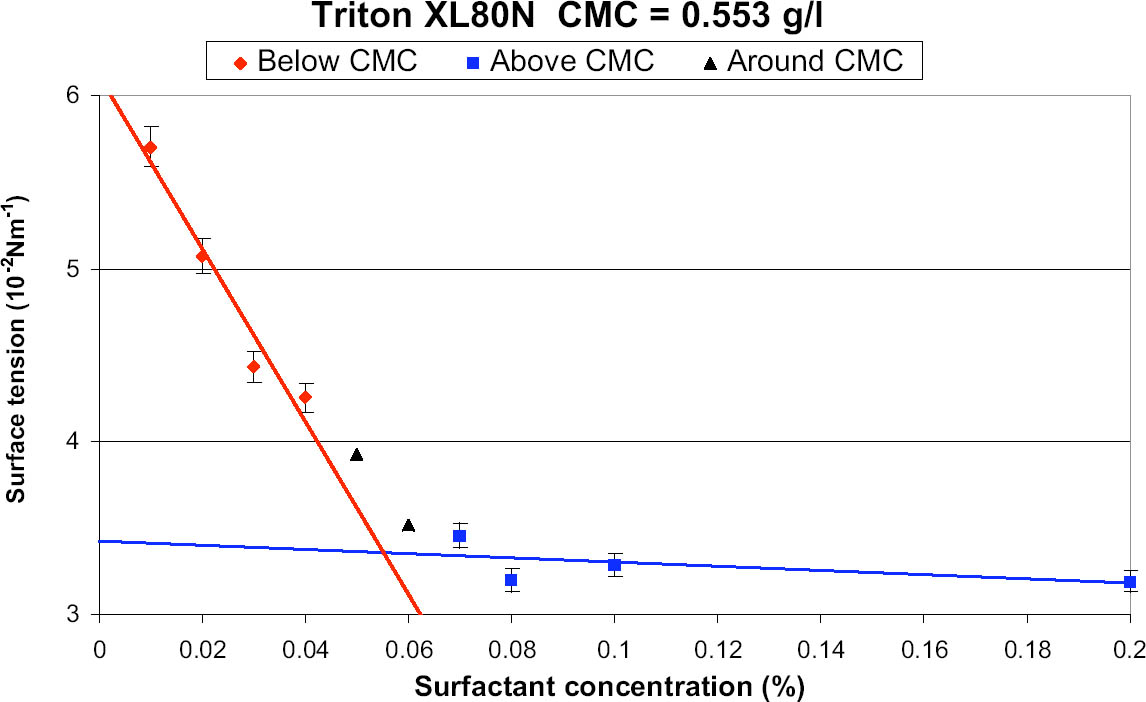
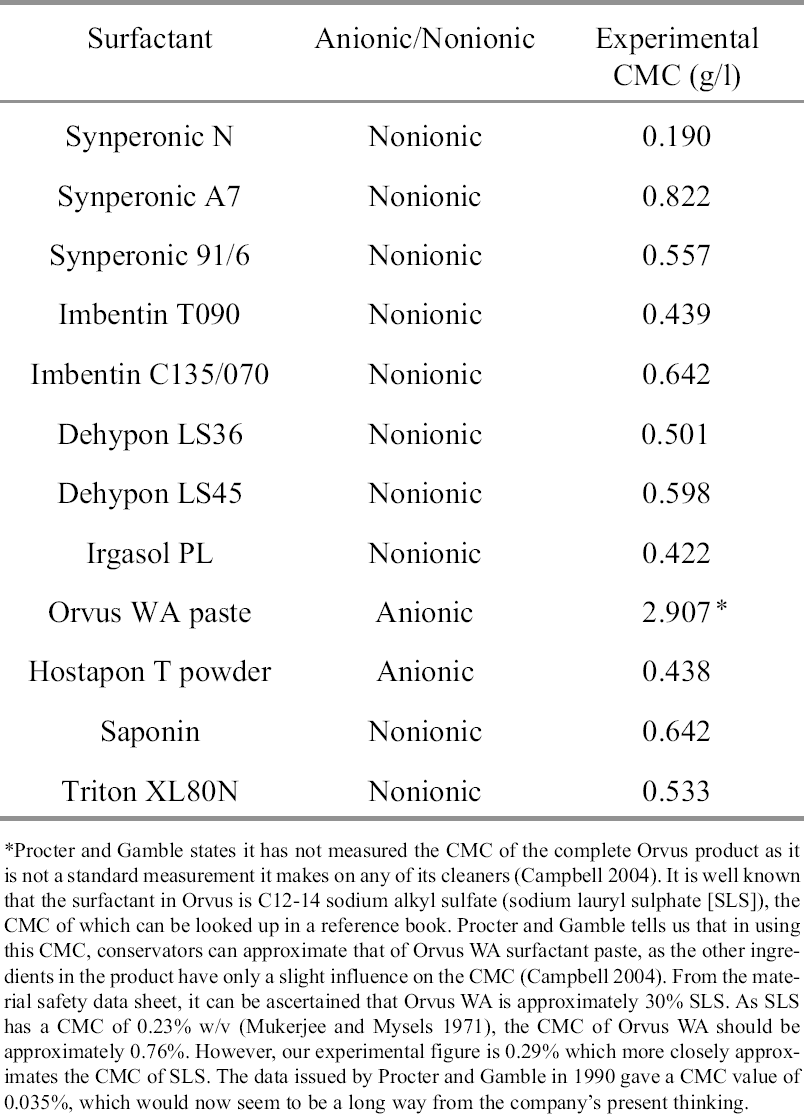
A graph of % w/v concentration vs. surface tension (γ) yields two straight lines with an intercept at the CMC (fig.3). The CMC for each of the surfactants was calculated in this manner at room temperature (20�C). The resulting CMC values can be seen in table 1. 4 SELECTION OF SURFACTANTS FOR TESTINGAfter consultation with conservators and the conservation literature, a list of surfactants suitable for testing was compiled by a working group that included the authors and two other members of the conservation department at the British Museum. Dr. Judy Daniels (Uniqema) advised on alternatives from the new Synperonic range. The initial list of 24 alternative surfactants was reduced to 11 with due consideration given to availability and the technical literature. The cleaning efficiency of distilled-water solutions of these 11 surfactants and Synperonic N were determined experimentally using distilled water as a control. The pH and conductivity of the wash liquors were also monitored.
4.1 EVALUATION CRITERIAAlong with its availability and expense, the other main factors that should be considered include efficiency of cleaning and residue (Hofenk de Graff 1968; Timar-Balazsy and Eastop 1998). 4.2 EFFICIENCY OF CLEANINGInitially the cleaning power of detergents is the most important consideration. The detergent should be capable of removing common dirt types and solubilizing waxes and greases. The efficiency of soil removal can be investigated by measuring the brightness/color of the samples before and after washing. 4.3 RESIDUEThere should be no residue of detergent deposited on the surface of the textile. To achieve this result, detergents with low fiber affinity are used, although this quality often reduces washing efficiency. Residues may be directly observed using scanning electron microscopy or measured quantitatively using time-of-flight secondary ion mass spectrometry (Howell and Carr 2000). These techniques were not used in this study, as we were not directly interested in determining the actual residues on the textile. Instead, the levels of water-soluble acids and ionic species were monitored by following the pH and conductivity of the wash water. 5 PHASE 1: EVALUATION OF THE SURFACTANTSThis first set of experiments aimed to provide a subset of approximately six surfactants for further testing. The selection criteria were based chiefly on efficiency of cleaning, taking into account the conductivity and pH of the washing solutions as indications of rinsing efficiency. To determine the cleaning efficiency, the color change was measured after experimental washes were performed on standard soiled wool and cotton fabrics. Many standard soiled fabrics are commercially available; those used for these experiments were manufactured by Testfabrics. The soiling had an initial composition of keltex 1.3%, corn starch 2.2%, water 72.4%, mineral oil 14%, oleic acid 0.4%, butanol 0.3%, morpholine 0.4%, vegetable fat 1.7%, solvesso 4.5%, ethyl cellulose 0.7%, and carbon black 0.7%. The fabric comes in a strip half printed with the standard soil (fig. 4). Rolls of both wool (style 530) and cotton (style 405) textile were obtained. These fabrics were kept in a refrigerator when not in use to prevent mold growth. A textile conservator devised a standardized manual cleaning technique so that it reflected the standard cleaning regime that would apply to a textile (table 2). The use of a mechanical shaker was investigated instead of using manual sponging of the object. By lengthening the washing time, the mechanical cleaning regime produced approximately the same color-change results as the manual hand washing (table 3). The mechanical regime was used in the first round of tests, and the manual method in the second round.
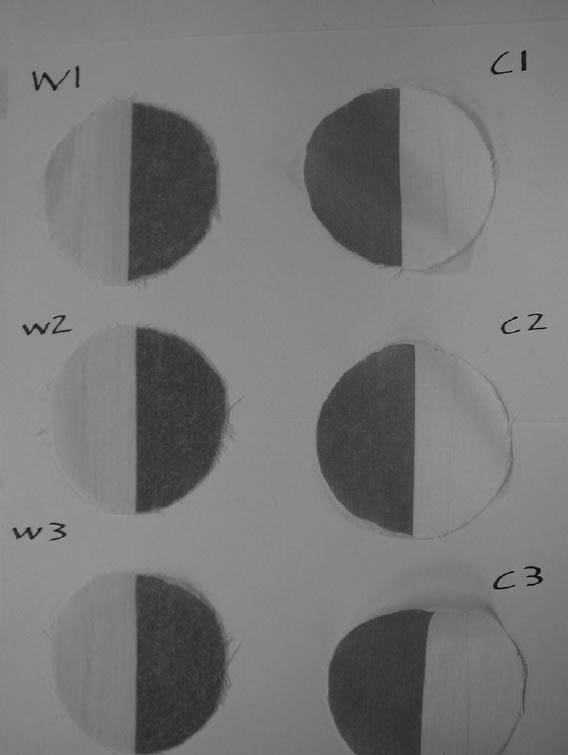
Discs of test fabric (80 mm diameter) were cut from the wool and cotton fabrics so that half of the disc was clean and the other half soiled (see fig. 4). Using a Minolta CR-300 Chroma Meter, which was calibrated using the standard tile provided by the manufacturer, the color of the surface was measured five times at three points on each half of the fabric using a Melinex template to locate the measuring areas. Measurements of the slightly translucent textiles were obtained in the CIE1976 L*a*b system with the textile resting on a white glazed tile. The textile discs were then placed faceup in a 250 cm3conical flask with a screw-top stopper, and 100 cm3of the test solution was added. The washing followed the regime described in table 2.
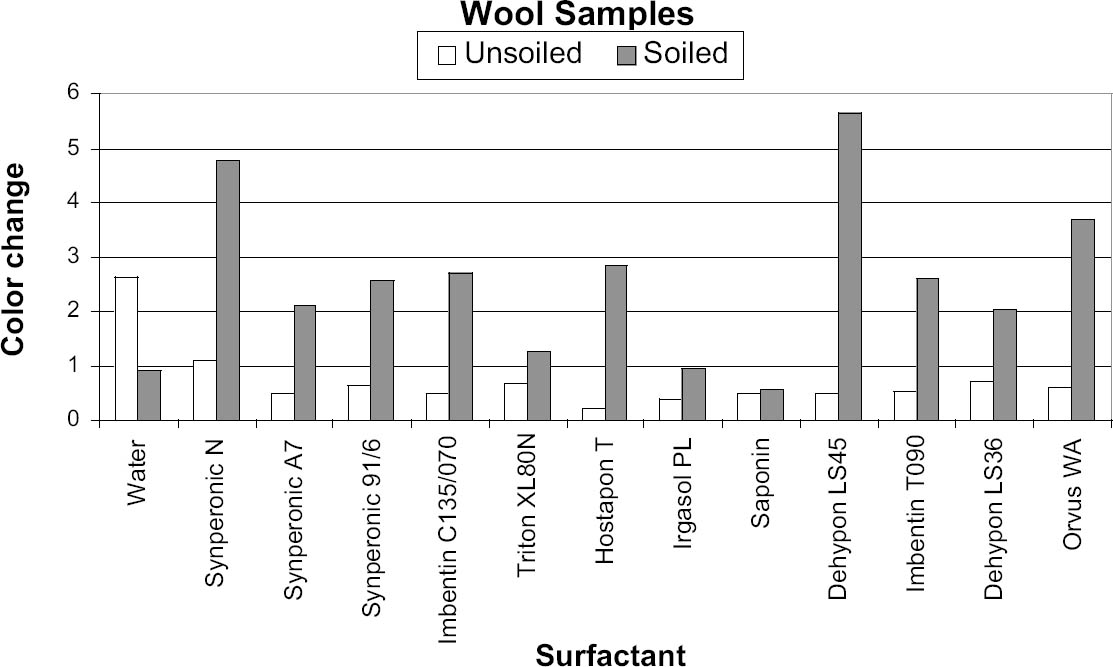
As the solutions were emptied from the flasks, 50 cm3 was saved for pH and conductivity measurements. The fabric discs were placed on a Melinex sheet to dry overnight before the color was remeasured as described above. 6 RESULTS FROM PHASE 16.1 CONDUCTIVITYThe conductivity behavior of solution samples obtained during the cleaning of the model textiles was similar whether wool or cotton was being washed. The conductivity of samples from nonionic cleaning was low-ranging from 7.9 to 38.4 μS, and showed a gradual decrease during rinsing. The two anionic surfactants gave higher conductivity readings at around 185 μS, which similarly decreased on rinsing. 6.2 pHThe pH readings ranged from 4.03 to 7.25; thus some readings fell outside the range expected for the washing of cotton, pH 5.5–8.0 (BSI 2002) and wool, pH 5.0–7.0 (Timar-Balazsy and Eastop 1998). Triton XL80N and Synperonic N consistently produced the lowest pH solutions. Triton XL80N samples varied from 4.03 to 6.36, while Synperonic N samples varied between 4.04 and 6.46. Otherwise the pH readings fell within the acceptable limits. 6.3 COLORThe color change (ΔE) before and after washing was calculated using the formula
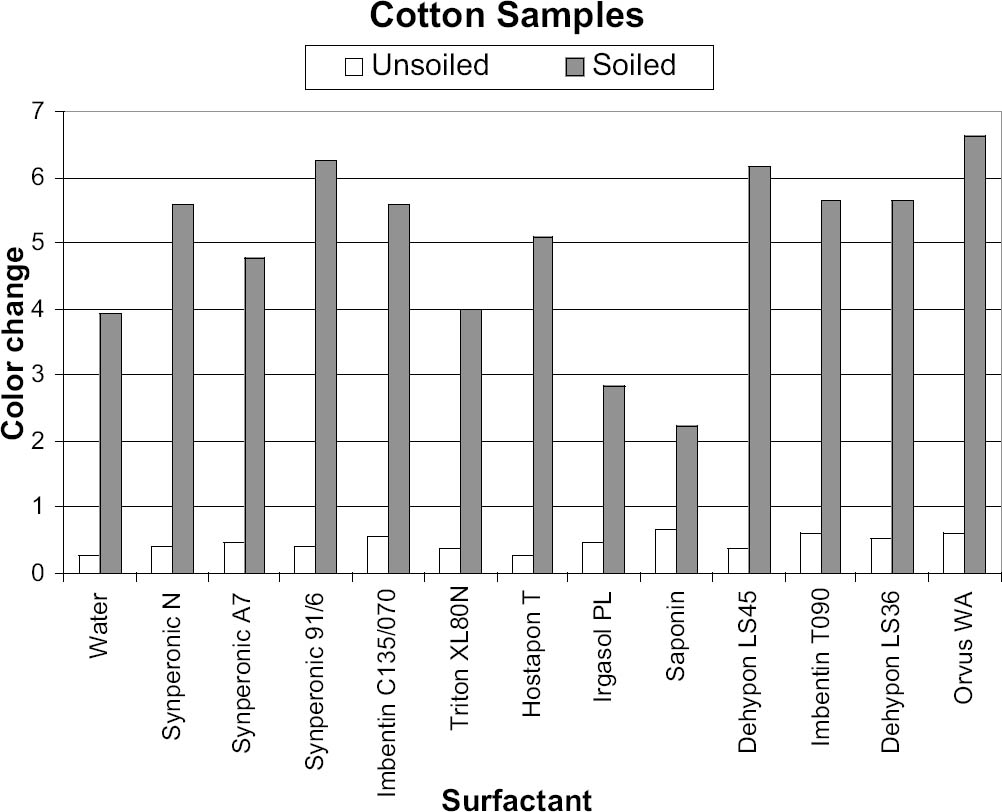
The results were transferred to two graphs for initial analysis, with each graph (figs. 5, 6) representing a particular fabric type. These graphs were useful, as they gave a direct indication of the cleaning ability of each surfactant, with the gray columns representing the soil liberation from the presoiled areas. The white column on figures 5 and 6 represents soil redeposition to the clean area, but this finding was only confirmed by reviewing the original data. The positive-negative nature of the ΔE value is lost when the root mean squared formula is applied. In all instances the values for color change of the soiled areas were opposite in sign to those of the unsoiled areas, representing soiling of the clean side through redeposition. In all cases the clean area of textile became darker due to redeposited soiling, while the dirty area became cleaner. 7 CONCLUSIONS FROM PHASE 1The pH of the wash water of all the surfactants except Triton XL80N was within the ranges discussed above, and therefore Triton XL80N was eliminated from further testing because of its low wash-water pH. The conductivity of the wash water of all the surfactants was so similar that no surfactants were further eliminated on this basis. With Triton XL80N eliminated, two members of the working group (Fields and Wingham) independently selected their best surfactants based on the color change (ΔE) before and after washing for both cotton and wool samples—those with the greatest change in ΔE for both wool and cotton. The combined list contained seven surfactants for further testing: five nonionic surfactants—Dehypon LS45 (liquid), Dehypon LS36 (liquid), Imbentin C135/070 (paste), Imbentin T090 (paste), and Synperonic 91/6 (liquid)—and two anionic surfactants—Hostapon T (powder) and Orvus WA (paste). For cotton, three surfactants—Dehypon LS45, Synperonic 91/6, and Hostapon T—proved equally most efficient at soil liberation. For wool, Dehypon LS45 was most efficient at soil liberation, followed by Hostapon T. Irgasol PL and Saponin were not brought forward for
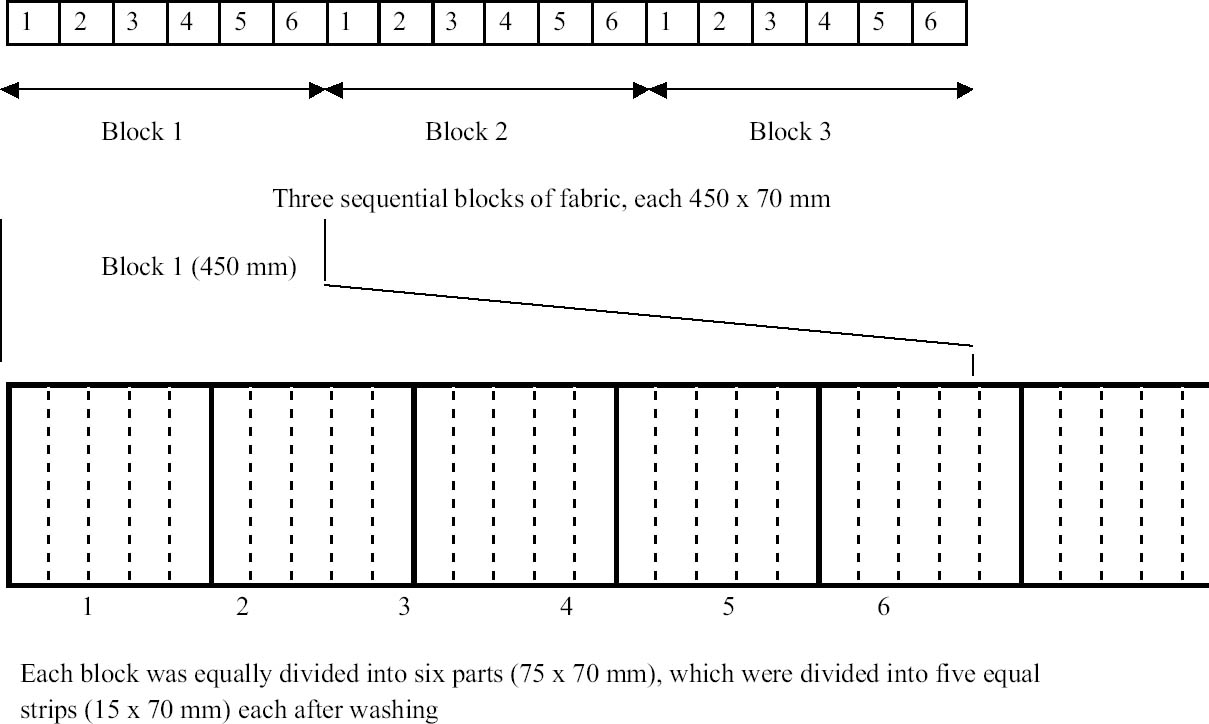
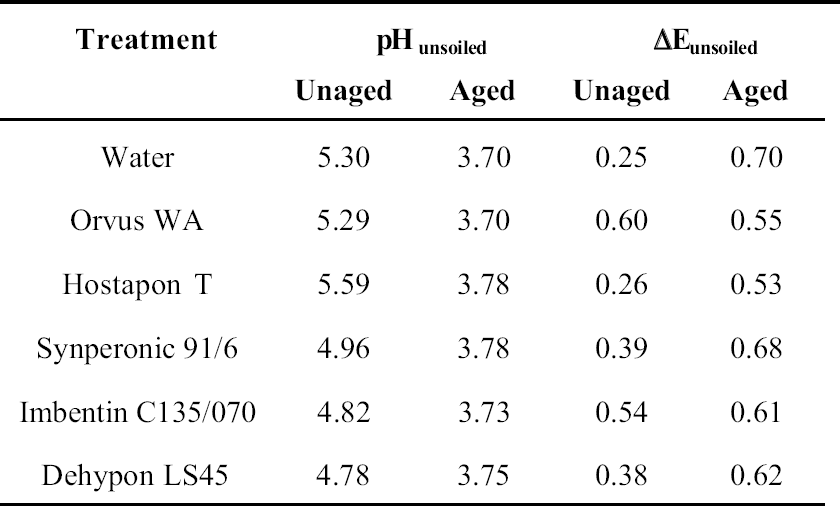
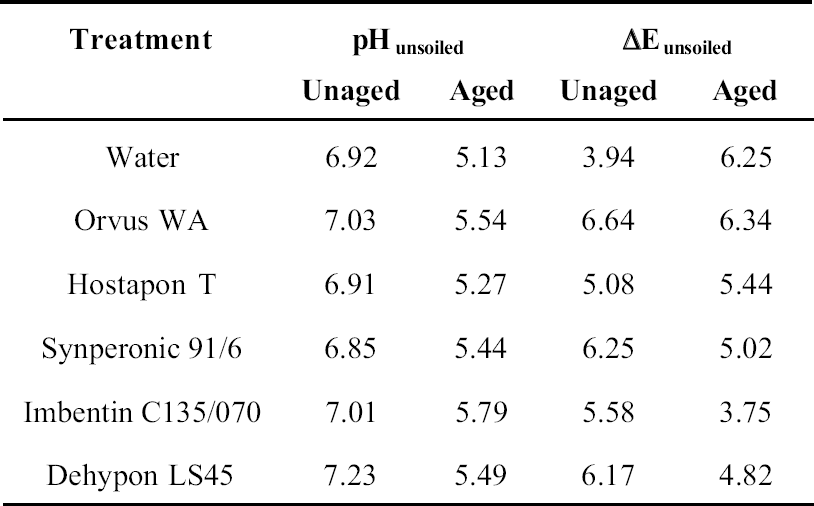
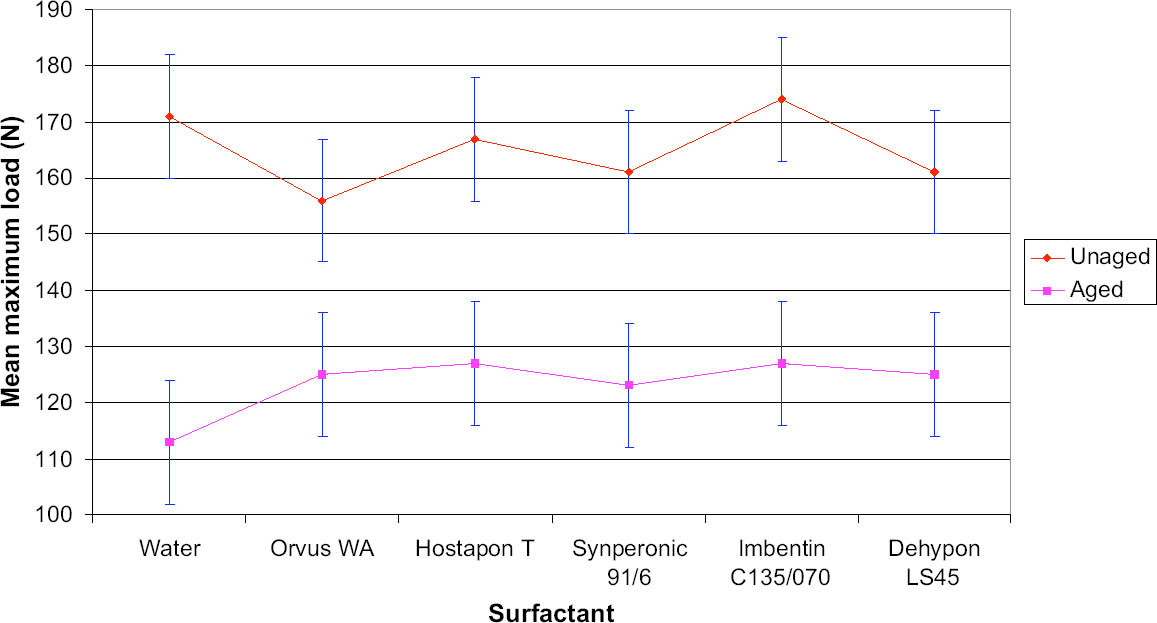
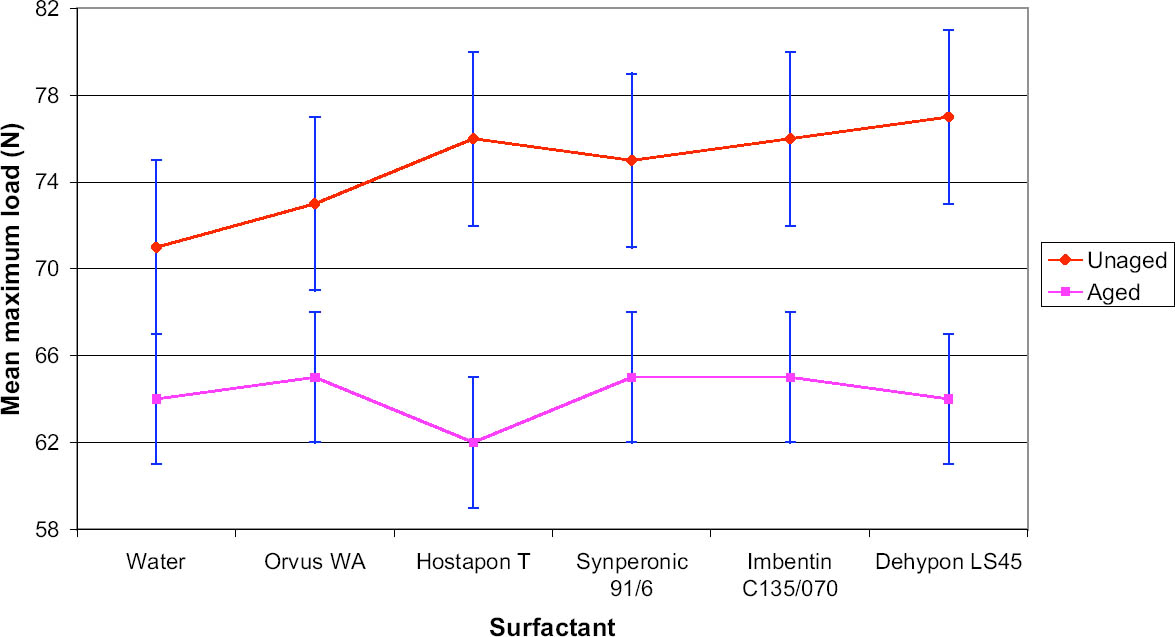
8 PHASE 2: FURTHER EVALUATION OF SELECTED SURFACTANTSThe seven best surfactants identified in the first phase of this study included two pairs of chemically similar nonionic surfactants from the same manufacturers: Imbentin T090 and C135/070 and Dehypon LS36 and LS45. Only one from each pair, Imbentin C135/070 and Dehypon LS45, were chosen for further testing with Synperonic 91/6 and two anionic surfactants, Hostapon T and Orvus WA. Phase 2 was to investigate whether artificially aged samples of surfactant-cleaned cotton and wool textile produced any effect that would cause a change in color or pH or reduce the tensile strength. The five surfactants listed above were tested with water as a control. Tensile testing was to be carried out on 15 strips each of the Testfabric cotton and wool textiles, each measuring 15 x 70 mm and cut parallel to the weft. To minimize any variation that might occur in the fabric, three sequential blocks of fabric measuring 450 x 70 mm were cut from the roll. Each of these blocks was equally divided into six parts and numbered 1–6 accordingly. All of the pieces numbered 1 were washed in Imbentin C135/070, those numbered 2 in Dehypon LS45, and so on, according to table 4, using the washing regime described in table 3. When washed and dried, the pieces were then cut into 5 equal strips, giving a total of 15 strips for each surfactant (fig. 7). These samples were then aged as described below. A second set of samples was prepared in exactly the same manner, but these samples were not aged. Color measurements on the cleaned samples were made as previously described, and these were aged in a light box fitted with Philips PL-L 36W compact fluorescent lamps (color 84, 2900 lumen, color temperature 4000 K) (Daniels and McIntyre 1993). One of the fans in the box was disabled to give elevated temperatures (53–58�C). The illuminance
For tensile testing, the samples were cut using a scalpel into strips measuring 15 x 70 mm, as described above. Care was taken not to cut warp threads parallel to the new cut. For the wool strips, each strip was prepared with 31 whole-warp threads, which was achieved by pulling out every 32d thread and cutting in the gap produced. This process was not possible with the cotton, as the threads were too firmly held to pull out. Samples were preconditioned at 60% RH for at least 24 hours before testing in accordance with British Standard BS4303 (BSI 1968). Tensile testing was carried out on an Instron 4411 with the interjaw distance at 40 mm and the extension rate at 20 mm/min. The maximum load was measured. The broken samples were retained for pH testing. The pH of samples before and after testing was measured by immersing a 1.0 g sample in freshly boiled and cooled deionized water (50 cm3). After an hour, the pH was measured using a combination glass electrode. 9 RESULTS OF PHASE 2 TESTING9.1 COLOR CHANGEThe cotton samples changed color least during light-aging, and there was no significant difference between samples washed with the different surfactants (tables 5, 6). In contrast, the wool yellowed to an extent that was easily seen by the naked eye, although the differences between samples was not significant. Measuring the color change data for the wool samples indicated that the sample treated with Orvus yellowed most, followed by the sample treated only with water. Imbentin showed the least yellowing. 9.2 TENSILE STRENGTH CHANGEBoth the wool and cotton samples showed a significant weakening after aging, but there were no significant differences between the surfactant treatments. All values lay within one standard deviation of the mean (figs. 8, 9). 9.3 pHAging produced a decrease in pH in all samples, but the use of the surfactants did not produce any significant further decrease in pH relative to water (see tables 5, 6). 10 CONCLUSIONS FROM PHASE 2The results for color change allowed several factors to be highlighted. The variation in color measurements for wool was minimal. It was apparent from the readings how easily and rapidly woolen fabrics can discolor. The variation between the cotton results was in all cases within the bounds of error, and thus they were discounted. The tensile testing results illustrated variations between aged and unaged samples in the case of both cotton and woolen fabrics. The results yielded very limited information with which to distinguish between the individual surfactants and their effects on the strength reduction. It was clear that no individual
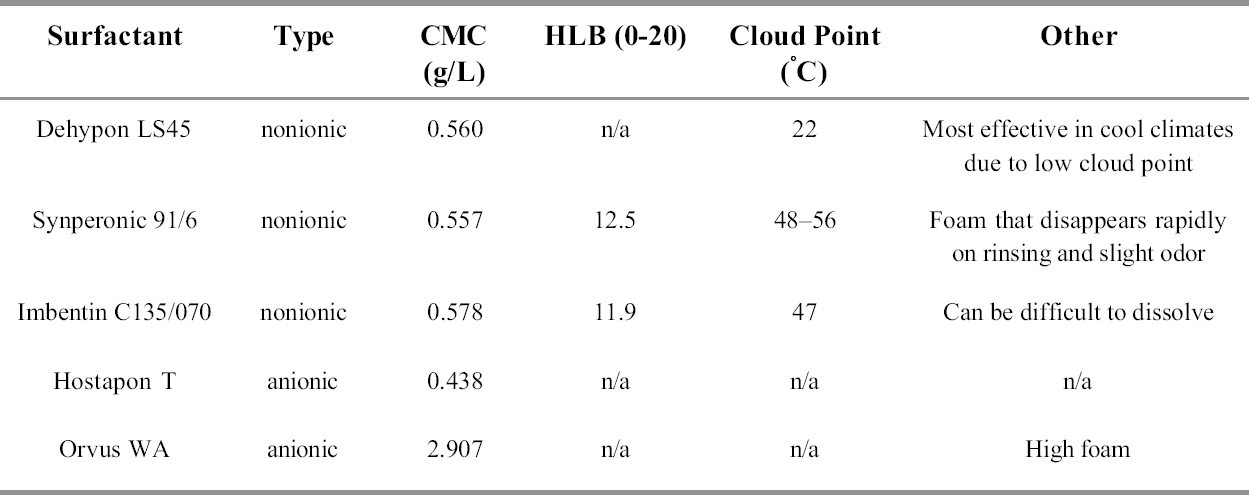
11 DISCUSSIONWithdrawal of the use of Synperonic N has made us consider the properties demanded of a generalpurpose conservation surfactant. The present selection has been based mainly on cleaning ability of the pure surfactant in distilled water solution acting on standard soiled textiles. Better cleaning could, no doubt, be obtained by adding performance-enhancing additives such as a suspending or chelating agent, but the ranking of the surfactants could then be altered. It is important to note that the experiments do not reveal whether any residual surfactant remains behind after rinsing. In most but not all cases, significantly more soiling is removed where a surfactant is used in the washing liquid, i.e., more than water alone. Aging of the washed model textiles did not show enhanced deterioration compared with water alone. Residues may remain after rinsing because of porosity—e.g., stone, ceramics, or metals—or, in the case of anionic surfactants, because of deposition of an insoluble metal soap. Although not experimentally evaluated in this study, it should be noted that if multivalent metal ions are present in significant quantities, the nonionic surfactants might be the better choice. Users may find that some of the five surfactants appear to have little to distinguish them in the tests; some of them have peculiarities that may make users prefer one to the others. Orvus WA has a high level of foam, Synperonic 91/6 has foam that disappears rapidly on rinsing and a slight odor, Imbentin C135/070 can be difficult to dissolve, and Dehypon LS45 often produces a cloudy solution in water. Dehypon's cloud point (at 1% concentration or at 5 x CMC = 0.3%) is 20–22�C; thus, in warm weather, Dehypon produces a cloudy solution and in cold weather a clear solution. The cleaning action of nonionic surfactants is greatest around the cloud point (Jakobi and L�hr 1987) but decreases rapidly above the cloud point, until at 5�C above it there is effectively no surfactant action. Thus, Dehypon LS45 should not be used at too high a temperature. A summary of the data can be seen in table 7. 12 CONCLUSIONSEvaluation of the results of the washing studies shows that five surfactants are suitable as substitutes for Synperonic N. These are the anionic surfactants Hostapon T and Orvus WA paste and the nonionic surfactants Dehypon LS45, Imbentin C135/070, and
13 ECOLOGICAL LABELING OF SURFACTANTSSurfactants by their very nature are harmful to living things, as they alter the properties of membranes surrounding cells. The European Chemicals Hazard Information and Packing (CHIP) for supply regulations require labeling of materials that are potentially ecologically dangerous. Products with a concentration that is lethal to 50% of the population of a given species (LC50) at less than 1 mg per L must bear a label with a dead fish and tree on it (fig. 10) and the associated R50 risk phrase:“This product will cause damage to the environment.” Containers of surfactants Synperonic N, Imbentin 136/070, and Dehypon LS45 should have this label. However, Synperonic 91/6 has an LC50 of 10–100 mg per L and does not bear this label. It has been noticed that when replacement surfactants have been given to conservators for trial, many do not wish to use the products with the dead-fish-and-tree label. These conservators may prefer to use Synperonic 91/6. The apparent importance of the labeling problem is diminished when it is realized that the labeling refers to surfactant discharged straight into water courses, although, in practice, water from workshops first goes into sewage treatment plants where surfactants are deactivated by biodeterioration. Thus, the choice of a surfactant from those recommended here does not need to be influenced by labeling requirements. ACKNOWLEDGEMENTSWe would like to thank Susan Bradley (head of Conservation Research, British Museum) for her support and encouragement; Dr. Judy Daniels of Uniqema for her time and her knowledge of the surfactant industry; Pippa Cruikshank (organics conservator, British Museum) for assisting in evaluations; David Howell (conservation scientist, Royal Historic Palaces) for the loan of the shaker; and Professor John Seddon (Department of Chemistry, Imperial College, London) for the facilities to perform the CMC experiments. Many of the results from this work, including data on all 24 surfactants tested and background information, can be found in Andrew Wingham's dissertation, which is published, in part, at www.collectablegifts.net/synperonic/synperonic.htm.
APPENDIXAPPENDIX 1Definitions Cloud point: temperature below which the solubility of an anionic surfactant drops dramatically and above which the solubility of a nonionic surfactant drops dramatically. When the surfactant comes out of solution, it forms a white cloud of minute particles. The nominal cloud point is measured on a 1% w/v aqueous solution. Nonionic surfactants have their greatest surface activity near the cloud point (Timar-Balazsy and Eastop 1998). Critical micelle concentration (CMC): surfactants form aggregates of molecules or ions called micelles when the concentration of surfactant solute in the bulk of the solution exceeds a limiting value, i.e., the critical micelle concentration. The CMC is a fundamental characteristic of each solute and is also temperature dependent (Howe-Grant 1977). Hydrophile-lipophile balance (HLB): a means for characterizing surfactants based upon the proportion of hydrophilic to lipophilic regions. An HLB value of zero represents a water-insoluble surfactant. The maximum HLB value is 20, although most surfactants tend to lie in the region of 11 to 15. The value for the HLB can be calculated on the basis of its molecular weight. One such method for ethoxylates is a fifth of the percentage by weight of ethoxylate in the molecule (Daniels 2000). REFERENCESBSI. 1968. British Standard for determination of the resistance to tearing of woven fabrics by the wing-rip technique, BS 4303. London: British Standards Institute. BSI. 2002. British Standard for repair and allied processes for the conservation of documents— recommendations, BS 4971. London: British Standards Institute. Campbell, B.2004. Personal communication. Product Development, Commercial Product Development Group, CPG TN6, Procter & Gamble, Cincinnati, Ohio. Daniels, J.2000. Synperonic N replacement. Unpublished report and presentation. Uniqema, Middlesborough, U. K. Daniels, V., and I.McIntyre. 1993. An apparatus for studying conservation light bleaching. Conservation Science in the United Kingdom, ed. N. H.Tennent. London: James & James. 122–24. Gentle, N., and S.Muller. 1995. An initial study of detergents and washing recipes for use in the conservation of textile objects. Conservation News58:55–59. Hofenk de Graff, J. H.1968. Constitution of surfactants in conjunction with the cleaning of ancient textiles. Studies in Conservation13:122–41. Howe-Grant, M., ed. 1977. Encyclopedia of Chemical Technology23:478. Howell, D., and C.Carr. 2000. Personal communication. Historic Royal Palaces, Textile Conservation Studio, Surrey, U. K., and School of Chemistry, University of Manchester Institute of Science and Technology, Manchester, U. K. Jakobi, G. and A.L�hr. 1987. Detergents and textile washing. Weinheim: VCH Verlaggsgesellschaft. Jobling, S., and J. P.Sumpter. 1993. Detergent components in sewage effluent are weakly oestrogenic to fish: An in vitro study using rainbow trout (Oncorhynchus mykiss) hepatocytes. Aquatic Toxicology27(3–4):361–72. Jobling, S., D.Sheahan, J. A.Osborn, P.Mattiessen, and J. P.Sumpter. 1996. Inhibition of testicular growth in rainbow trout (Oncorhynchus mykiss) exposed to estrogenic alkylphenolic chemicals. Environmental Toxicology and Chemistry15:194–202.
Lewis, J., and D.Eastop. 2001. Mixtures of anionic and non-ionic surfactants for wet-cleaning historic textiles: A preliminary evaluation with standard soiled wool and cotton test fabrics. Conservator25:73–89. Mukerjee, P., and K. J.Mysels. 1971. Critical micelle concentrations of aqueous surfactant systems. Washington, D. C.: U. S. Department of Commerce, National Bureau of Standards. Plenderleith, H. J.1956. The conservation of antiquities and works of art. London: Oxford University Press. Sugden, S.1922. The determination of surface tension from the maximum pressure in bubbles. Journal of the Chemical Society121:858–66. Sugden, S.1924. The determination of surface tension from the maximum pressure in bubbles. Part 2. Journal of the Chemical Society125:27–31. Timar-Balazsy, A., and Eastop, D.1998. Chemical principles of textile conservation. London: Butterworth-Heinemann Ltd. FURTHER READINGOSPAR Commission Report. 1998. DIFF98/9/1-E. Helsinki, Finland. SOURCES OF MATERIALSFabricsTestfabrics 100% wool (Style 530) and 100% cotton (Style 405) in a 10-yard roll approximately 9144 mm length x 100 mm wide with soiled portion approximately 437 mm length x 91 mm wideWestlairds Limited Patrixbourne The Green, Datchet Slough SL3 9JH, U.K. InstrumentsMinolta CR-300 Chroma Meter, 8 mm measuring area and 0o viewing angleMinolta (UK) Limited 1-3 Tanners Drive Blakelands North Milton Keynes MK14 5BU, U.K. EDT Instruments Series 3 pH meterEDT Instruments Dover, Kent CT16 2DR, U.K. CMD400 Digital Conductivity MeterWPA Milton Rd. Unit 22 Cambridge Science Park Cambridge CB4 0FJ, U.K. SurfactantsDehypon LS36, Dehypon LS45 Conservation Resources (UK) Ltd. Units 1, 2, 4 & 5 Pony Road Horspath Industrial Estate Cowley, Oxford OX4 2RD, U.K. +44-1865-747755 Hostapon TClariant GmbH c/o Chemlink Specialties Ltd. Carrington Business Park, Carrington Urmston Manchester M31 4ZU, U.K. +44-161-7764303 Imbentin T/090, Imbentin C/135/070Libra Chemical Ltd. Brinell Drive, North Bank Industrial Park, Irlam Manchester M44 5LF, U.K. +44-161-7751 888 Irgasol PLCiba Specialty Chemicals Plc. Charter Way Macclesfield SK10 2NX, U.K. +44-1625-421933 Preservation Equipment Ltd. Shelfhanger Diss Norfolk IP22 2DG, U.K. +44-1379-651527 SaponinSigma-Aldrich Chemicals The Old Brickyard New Road, Gillingham Dorset SP8 4XT, U.K. + 44-800-717181 Synperonic N, Synperonic A7, and Synperonic 91/6Uniqema P.O. Box 90 Wilton Middlesborough Cleveland TS90 8JE, U.K. +44-1642-432 395 Triton XL-80NUnion Carbide Benelux c/o SurfaChem Ltd. Wellington Park House, Thirsk Row Leeds, LS1 4DF, U.K. +44-113-2342636 Technical dataCMCs were experimentally determined by the authors, but the cloud points are from the manufacturer's literature. AUTHOR INFORMATIONJOHN A. FIELDS was awarded a B.Sc. in applied chemistry from Dublin City University in 1995, which he followed with a one-year internship in the Paper Conservation Studio at the National Gallery of Ireland. This work subsequently led to a Ph.D. on “Analysis of Ancient and Modern Chinese Paper” at Queen's University, Belfast, in conjunction with the British Library. Fields joined the British Museum in 1999 as a conservation scientist and worked mainly on conservation science problems relating to organic materials. In April 2001 he joined the Irish State Laboratory, where he is continuing to work in the area of heritage and environment. Address: The State Laboratory, Abbotstown, Dublin 15, Republic of Ireland; e-mail: john.fields@statelab.ie ANDREW WINGHAM received an M.Sci. in chemistry with conservation science at Imperial College London in conjunction with the Royal College of Art and the Victoria and Albert Museum. During his degree, he concentrated on analytical techniques and numerous conservation science issues, and for his dissertation he carried out research into the alternatives to Synperonic N. He has recently completed an M.Sc. in forensic archaeological science at the Institute of Archaeology, University College, London. Address: 1–3 High St., Biddenden, Kent TN27 8AL, U.K.; e-mail: andrew@collectablegifts.net FRANCES HARTOG began her three-year apprenticeship training in textile conservation in 1989 at the Textile Conservation Studio in London. From 1992 to 1994 she worked at the Textile Conservation Center, then undertook two years of contract work at the Museum of London. In 1996 she joined the National Trust at its Textile Conservation Studio in Norfolk. Two years later she took up her present post as a senior textile conservator in the Conservation Department of the Victoria and Albert Museum. Address: Textile Conservation, Victoria & Albert Museum, South Kensington, London SW7 2RL, U.K.; e-mail: f.hartog@vam.ac.uk VINCENT DANIELS studied chemistry for his B.Sc. and studied the reactions of thermally degraded polyvinylchloride (PVC) for his Ph.D. from University College, Cardiff, Wales. He joined the British Museum in 1974 to work in the Research Laboratory on the conservation science of paper and books. He joined the Department of Conservation on its formation in 1975. Since then he has worked on a wide variety of conservation science problems, but recurring themes are the deterioration of cellulosic fibers, dyes, and pigments. He left the British Museum in 2003 and is now a research fellow at the Royal College of Art. Address: Conservation Department, School of Humanities, Royal College of Art, Kensington Gore, London SW7 2EU, U.K.; e-mail: vincent.daniels@rca.ac.uk
 Section Index Section Index |