THE INFLUENCE OF CONSERVATION TREATMENTS AND ENVIRONMENTAL STORAGE FACTORS ON CORROSION OF COPPER ALLOYS IN THE ANCIENT ATHENIAN AGORAALICE BOCCIA PATERAKIS
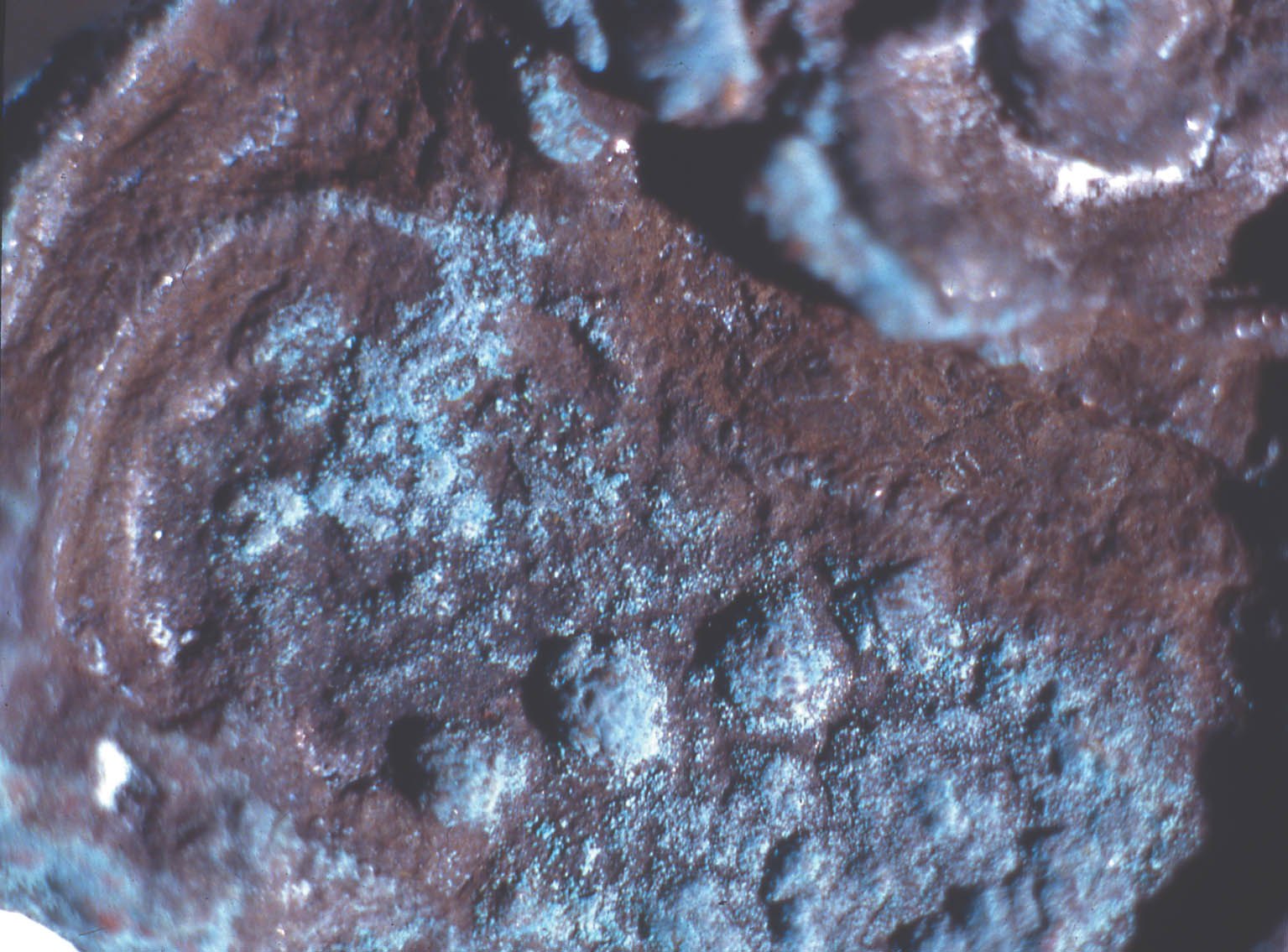
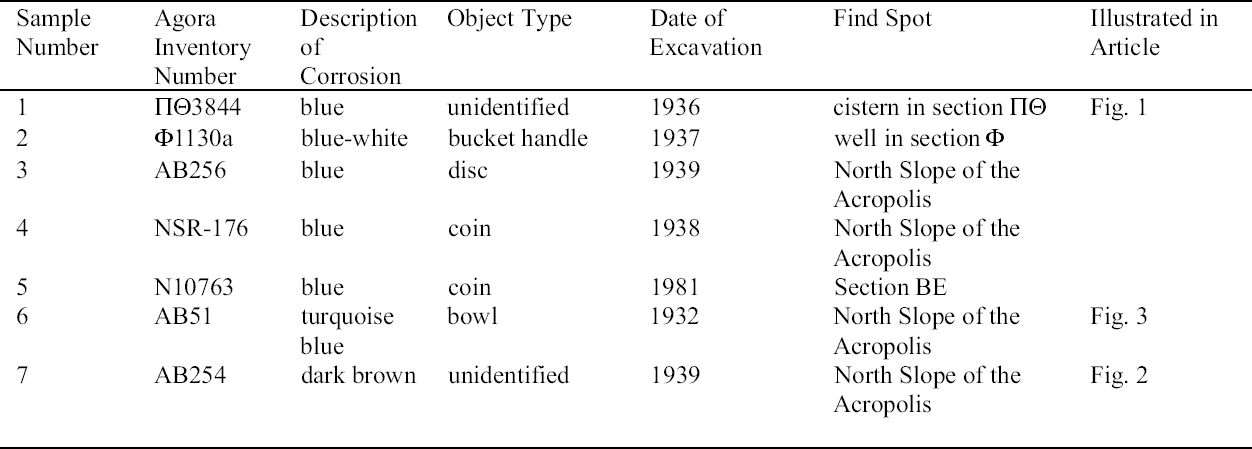
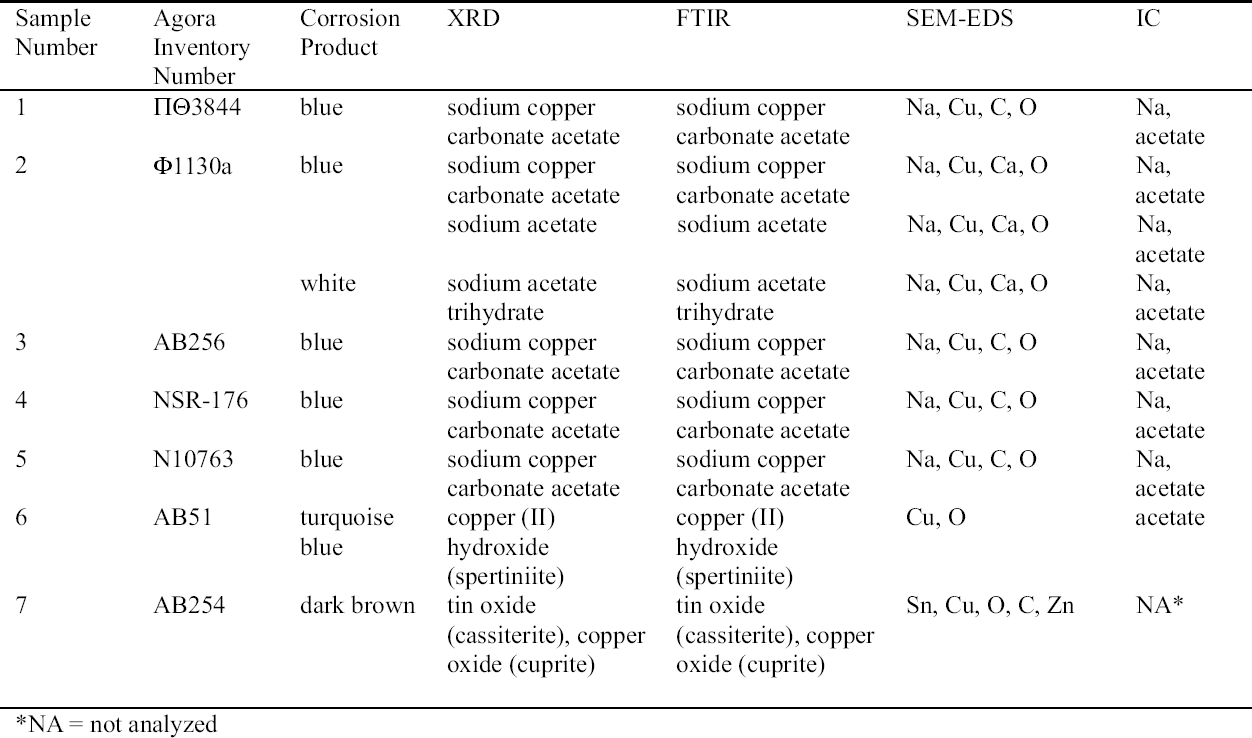
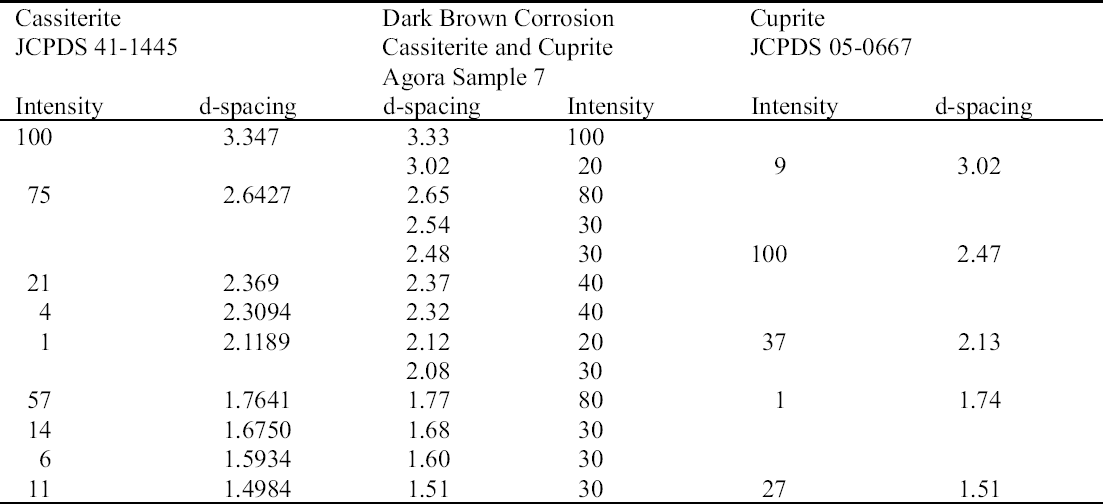
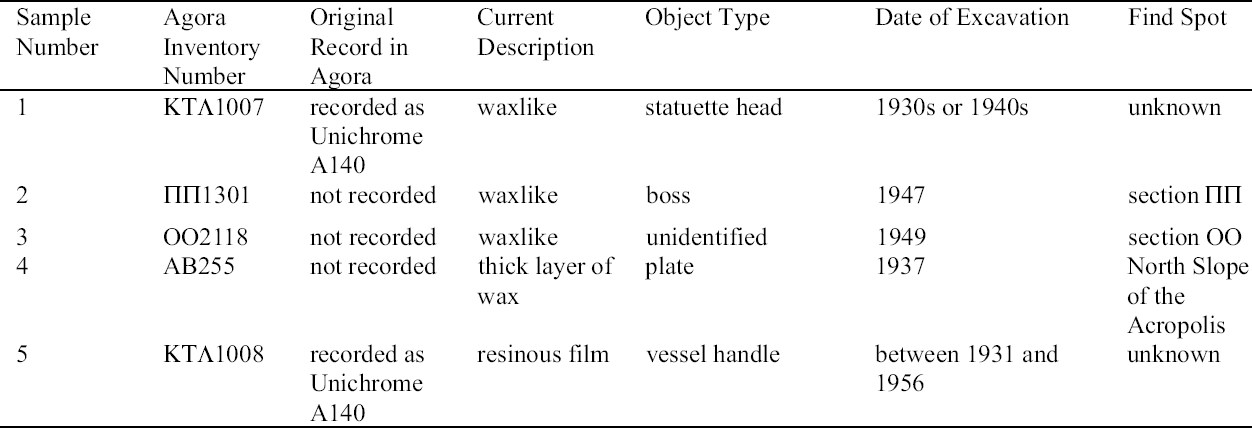
ABSTRACT—The Agora collection of copper alloys consists of mirrors, pins and needles, lamps, vases, tools, nails and other hardware, official weights, voting ballots, sculpture, jewelry, medical instruments, weapons, and coins excavated on the North Slope of the Acropolis and in the Ancient Agora of Athens, and is housed in the Stoa of Attalos on-site. Blue, turquoise blue, dark brown, and white corrosion products on these copper alloys have been identified as a sodium copper carbonate actetate, copper (II) hydroxide, copper and tin oxide, and sodium acetate trihydrate, respectively. The analysis of the corrosion was carried out by x-ray diffraction, Fourier transform infrared spectroscopy, scanning electron microscopy with energy dispersive x-ray microanalysis, and ion chromatography analysis. Conservation materials, as well as storage and environmental conditions, were found to play a role in the development of these corrosion products. Most of the objects have been chemically cleaned to various degrees. The role of cleaning and stabilizing agents containing sodium in creating the corrosion is considered. The contributing roles of acetic acid emissions from wooden storage materials and relative humidity are examined. Preliminary analysis of conservation coatings by FTIR was carried out to determine the effects of such coatings on the condition of the copper alloy collection. Solubilities of coatings used as lacquers and consolidants on the artifacts are reported. The coatings identified by FTIR were hydrocarbon waxes and cellulose nitrate. Observations regarding the conditions necessary for the development of corrosion are made. Recommendations for conservation and storage to prevent new corrosion or to alleviate further corrosion are given. TITRE—L'influence des traitements de restauration et des conditions ambiantes des r�serves sur la corrosion des alliages de cuivre provenant de l'ancienne Agora d'Ath�nes. R�SUM�—La collection d'alliages de cuivre provenant de l'Agora consiste en des miroirs, des �pingles et �pinglettes, des lampes, des vases, des outils, des clous et autres pi�ces de quin-caillerie, des poids r�glementaires, des bulletins de vote, des sculptures, des bijoux, des instruments m�dicaux, des armes et des pi�ces de monnaie. Ces vestiges, retrouv�s lors de fouilles de la pente nord de l'Acropole et de l'ancienne Agora d'Ath�nes, sont maintenant conserv�s sur le site m�me,� la Stoa d'Attalos. Des produits de corrosion bleus, bleu turquoise, brun fonc� et blancs sur ces articles ont �t� identifi�s comme �tant respectivement compos�s d'ac�tate et de carbonate de cuivre sodique, d'hydroxyde de cuivre (II), d'oxyde de cuivre et d'�tain, et finalement d'ac�tate trihydrat� de sodium. Ces analyses des produits de corrosion ont �t� faites par diffraction des rayons X, par spectroscopie infrarouge � transform�e de Fourier, par microscopie �lectronique � balayage coupl�e d'un dispositif de micro-analyse par rayons X � dispersion d'�nergie, et par analyse chromatographique d'�change d'ions. Il a �t� possible d'�tablir un lien entre l'�volution des produits de corrosion et les produits de conservation utilis�s, ainsi que les conditions des r�serves et du milieu ambiant. La plupart des artefacts ont �t� nettoy�s � divers degr�s dans le pass�. La possibilit� que des produits de nettoyage ou de stabilisation contenant du sodium aient jou� un r�le dans l'apparition de la corrosion est discut�e. De m�me, la part des �missions d'acide ac�tique provenant des �tag�res et autres mat�riaux de r�serve, ainsi que celle de l'humidit� relative, sont aussi examin�es. Des analyses pr�liminaires par spectroscopie infrarouge � transform�e de Fourier furent effectu�es pour d�terminer si divers produits de rev�tement utilis�s pour prot�ger les objets purent aussi contribuer � leur corrosion. La solubilit� de certains rev�tements et consolidants employ�s sur ces objets est rapport�e. L'analyse spectroscopique r�v�la que les rev�tements �taient des cires � base d'hydro-carbones et de nitrate de cellulose. Des observations sont pr�sent�es concernant les conditions n�cessaires pour le d�veloppement de la corrosion, et des recommandations sont �nonc�es concernant le traitement et la mise en r�serve de tels objets, dans le but d'�viter l'apparition de nouvelle corrosion et de freiner l'�volution de la corrosion existante. TITULO—La influencia de los tratamientos de conservaci�n y los factores ambientales de los dep�sitos sobre las aleaciones de cobre del antiguo �gora Ateniense. RESUMEN—La colecci�n del �gora de aleaciones de cobre consiste en espejos, broches y agujas, l�mparas, vasijas, herramientas, clavos y otros utensilios, pesos oficiales, boletas de votaci�n, esculturas, joyas, instrumentos m�dicos, armas, y monedas excavadas en la pendiente norte de la Acr�polis y en el antiguo �gora de Atenas, y se aloja en la Stoa de Atalos en la Acr�polis. Los productos de corrosi�n de color azul, azul turquesa, marr�n oscuro, y blanco en las aleaciones de cobre han sido identificados como sal doble carbonato acetato de cobre y sodio, hidr�x-ido c�prico, �xido doble de cobre y antimonio, y trihidrato de acetato y sodio respectivamente. El an�lisis de la corrosi�n se hizo por medio de la difrac-ci�n de rayos X (XRD), espectroscop�a infrarroja con transformada de Fourier (FTIR), microscop�a de barrido electr�nico con an�lisis de rayos X por energ�a dispersiva (SEM-EDX), y an�lisis de cromatograf�a i�nica. Se encontr� que tanto los materiales de conservaci�n como las condiciones del dep�sito y ambientales contribuyen al desarrollo de estos productos de corrosi�n. La mayor�a de los objetos hab�an sido previamente limpiados qu�micamente a diferentes grados de intensidad. Se discute el rol que tiene en la corrosi�n la limpieza y los agentes estabilizadores que contienen sodio. Se examinan los roles de las emisiones de �cido ac�tico, producto de los muebles de madera utilizados en el dep�sito, y de la humedad relativa. Se hace un an�lisis preliminar de los revestimientos (coatings) de conservaci�n por medio de FTIR para determinar los efectos de las capas protectoras en la condici�n de las aleaciones de cobre de la colecci�n. Se hace un informe sobre la solubilidad de las capas protectoras usadas, sobre los artefactos tales como lacas y consolidantes. Las capas protectoras identificadas por FTIR son ceras de hidrocarburos y nitrato de celulosa. Se hacen observaciones respecto a las condiciones necesarias para el desarrollo de la corrosi�n. Se hacen recomendaciones en cuanto a la conservaci�n y al dep�sito para prevenir nuevas corrosiones o para mitigar otras corrosiones adicionales. TITULO—A influ�ncia de tratamentos de conserva��o e os fatores ambientais de armazenagem na corros�o de ligas de cobre da antiga �gora Ateniense. RESUMO—A cole��o de �gora de ligas de cobre � composta de espelhos, alfinetes e agulhas, l�mpadas, vasos, ferramentas, pregos e outras ferragens, pesos oficiais, c�dulas de vota��o, esculturas, j�ias, instrumentos m�dicos, armas e moedas escavados na encosta norte da Acr�pole e na antiga �gora de Atenas, que se situa na Stoa de Attalos. Os produtos de corros�o de cor azul, azul turquesa, marrom escuro e branco nas ligas de cobre tem sido identificados respectivamente como carbonato acetato de cobre e s�dio, hidr�xido de cobre (II), �xido de cobre e estanho e tri-hidrato acetato de s�dio. A an�lise da corros�o foi feita por difra��o de raios X (XRD), espectroscopia infravermelha transformada Fourier (FTIR), microscopia de varredura eletr�nica com micro-an�lise de raios X por energia dispersiva (SEM-EDX) e an�lise de cromatografia i�nica. Chegouse � conclus�o que materiais de conserva��o, bem como armazenagem e condi��es ambi-entais, contribuem para o desenvolvimento dos produtos de corros�o. A maioria dos objetos tem sido limpa quimicamente em v�rios graus de intensidade. Na forma��o da corros�o, analisase o papel da limpeza e dos agentes de estabiliza��o que cont�m s�dio. Examinase a contribui��o da emiss�o de �cido ac�tico dos materiais de armazenagem feitos de madeira e a umidade relativa. Foram realizadas an�lises preliminares dos revestimentos de conserva��o usando FTIR para determinar os efeitos de tais revestimentos nas condi��es das cole��es de liga de cobre. S�o relatadas as solubilidades dos revestimentos usados nos artefatos tais como lacas e consolidantes. Os revestimentos identificados atrav�s de FTIR foram ceras de hidrocarbonato e nitrato de celulose. S�o feitas observa��es em rela��o �s condi��es necess�rias para o desenvolvimento de corros�o. S�o fornecidas recomenda��es para conserva��o e armazenagem para prevenir nova corros�o ou para minimizar corros�es adicionais. 1 INTRODUCTIONDuring a survey of the copper alloy objects excavated from the North Slope of the Acropolis in the 1930s, a blue corrosion was noted on many of them (Hall 1995). The blue material was often found on top of a dark brown corrosion layer and, in many cases, was intimately associated with a white crystalline corrosion. The blue corrosion is mossy in appearance. Since the survey, a more extensive investigation has been carried out on the cataloged copper alloy collections housed in the Agora, which consist of approximately 72,000 objects. The North Slope objects, which constitute 3.5% of that number, show blue corrosion on 20% of the coins and 40% of other objects. Significantly less than 20% of copper objects with this corrosion are observed in the other Agora collections. Blue corrosion on copper alloy objects from the North Slope was tentatively identified as a compound based on copper acetate by x-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FTIR) analysis (Paterakis 1998). The XRD patterns of five samples from five copper alloy objects were published for comparative purposes. In 1999 further analysis of the blue, the white, and the dark brown corrosion was carried out by XRD, FTIR, ion chromatography (IC), and scanning electron microscopy with energy dispersive x-ray micro-analysis (SEM-EDS). The blue corrosion was identified as a sodium copper carbonate acetate [NaCu(CO3)(CH3COO)], the white as sodium acetate trihydrate (CH3COONa.3H2O), and the dark brown as a copper oxide (cuprite, Cu2O) and tin oxide (cassiterite, SnO2) mixture. Blue corrosion on one object was identified as copper (II) hydroxide and shall be referred to as turquoise blue throughout this paper to distinguish it from the blue acetate compound (Paterakis 1999). Two sodium copper carbonate acetate compounds and sodium acetate trihydrate have been identified in the British Museum on Egyptian copper alloys stored in wooden cupboards since the 1930s (Thickett 1998; Thickett et al. 1998; Thickett and Odlyha 2000). A comparison of the corrosion present on these copper alloys with those in the Agora, which also have been kept in wooden storage cases since the 1930s, is consistent with similar published observations suggesting that the buildup of acetic (ethanoic) acid is a major contributor to the formation of the acetate corrosion products (Tennent and Baird 1992; Tennent et al. 1993). The Agora storage cases are constructed of softwood and thin plywood. In the Agora collections the blue corrosion has been found solely on chemically treated objects, whereas in the British Museum it was found also on objects that retained their original corrosion products. The purpose of this article is to present in some detail the results from the recent campaign of corrosion analysis on the Agora collection and to explore the role that conservation methods and materials, as well as environmental storage conditions, may have played in the formation of this corrosion. To this end, the collection was surveyed for corrosion and conservation coatings, a few coatings were selected for analysis by FTIR, and the preliminary results of FTIR analysis are presented. 2 ANALYSIS OF CORROSION PRODUCTSAnalytical means in addition to those used in 1998 were required in order to identify the corrosion compounds. Analysis of the turquoise blue, blue, dark brown, and white corrosion products was carried out by XRD, FTIR, IC, and SEM-EDS in 1999. 2.1 ANALYTICAL TECHNIQUESFor powder XRD, a Debye-Scherrer camera was used, which allows the analysis of samples as small as 1 �g. Analysis was undertaken using Cu (alpha) radiation produced from an x-ray tube operating at 40 keV and 40 mA. A Phillips PW1120/90 generator was used. The samples were ground and adhered to a gelatin strip by wetting the end in distilled water and brushing it over the samples. FTIR characterizes the vibrational transitions of a material. It complements XRD in that it can be used for amorphous as well as crystalline materials. The use of a beam condenser and diamond cell allows analysis of sample sizes similar to those used for powder XRD. A Spectratech Sample Plan diamond cell with a 4x beam condensor on a Nicolet Ion chromatography is a modification of liquid chromatography permitting simultaneous determination of many anions and cations in solution, including acetate. Analysis was undertaken with a Dionex DX300 system using an AS12A column with 2.7 mM sodium carbonate and 0.3 mM sodium bicar-bonate eluant for anions and a CS12 column with 20 mM methane disulfonic acid eluant for cations. SEM-EDS can generate elemental analysis from extremely small samples (1 � diameter). It can be sensitive and quantitative for all elements except boron and hydrogen. The samples were pressed onto a conductive carbon pad and analyzed uncoated. The very small sample size and carbon pad allowed qualitative analysis without coating. A Joel 840 SEM with Link analyzer system was used for this work. The accelerating voltage was 25 kV and the current was 1 nA. 2.2 CORROSION SAMPLED FROM COPPER ALLOY OBJECTSThe objects sampled for corrosion and a description of this corrosion are presented in table 1. The objects from which samples 1, 2, 3, 4, 5, and 7 were removed had been chemically cleaned. Although the cleaning method used on these objects was not recorded, electrochemical reduction with zinc and sodium hydroxide is likely, based on the resulting dark, powdery, reduced corrosion (see sec. 3.3). Dark brown corrosion underlies the blue corrosion on samples 1, 2, 3, 4, and 5. White crystals coexist with the blue corrosion in varying quantities (fig. 1, see page 257). The dark brown corrosion (sample 7) consists of a soft powder that was easily removed from the surface (fig. 2, see page 258). The blue corrosion associated with it was also easily removed with a scalpel. The turquoise blue corrosion (sample 6) formed on top of malachite and cuprite of the completely mineralized bowl. With these other corrosion products, the turquoise blue corrosion forms a crust that is friable and easily flakes off, leaving a smooth surface underneath. The blue is intimately bound with the underlying corrosion and could not be sampled without removing the substrate. Although the bowl does not appear to have been chemically cleaned, it may have been treated with sodium sesquicarbonate (see sec. 3.1.2.4) (fig. 3, see page 259).
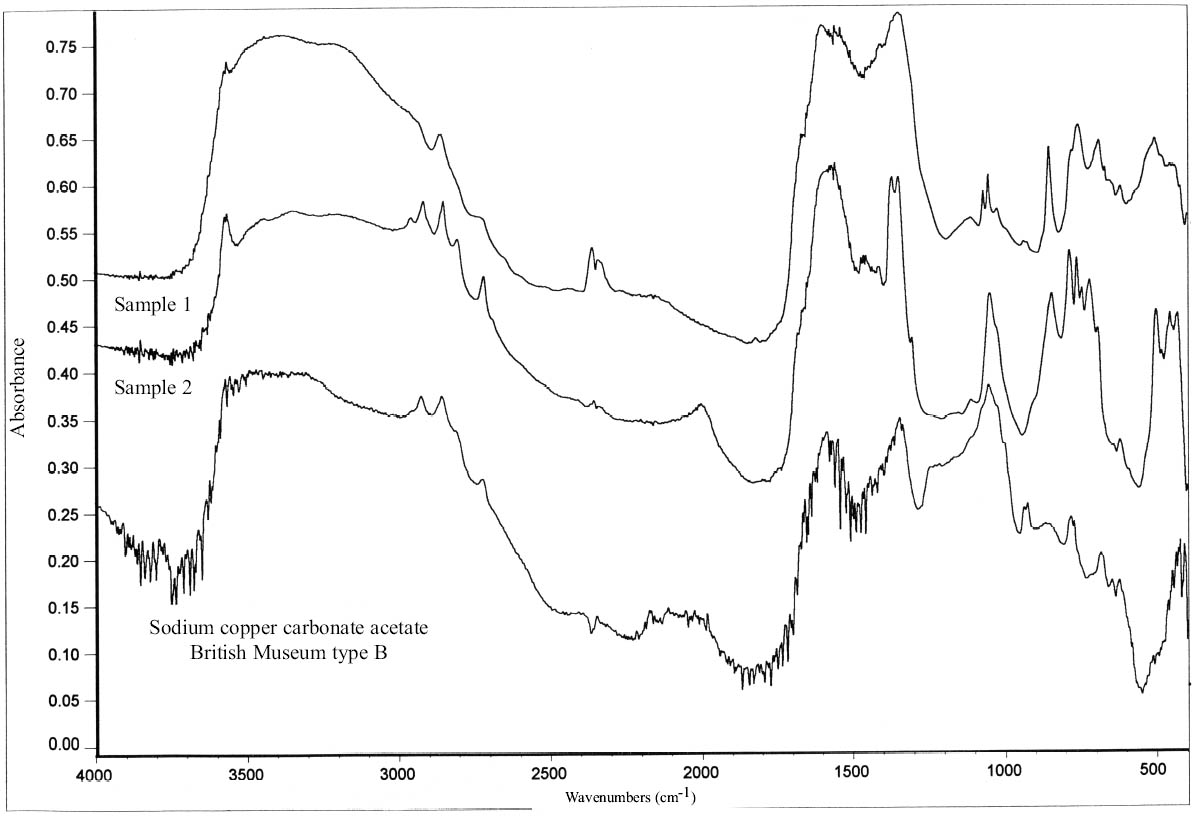
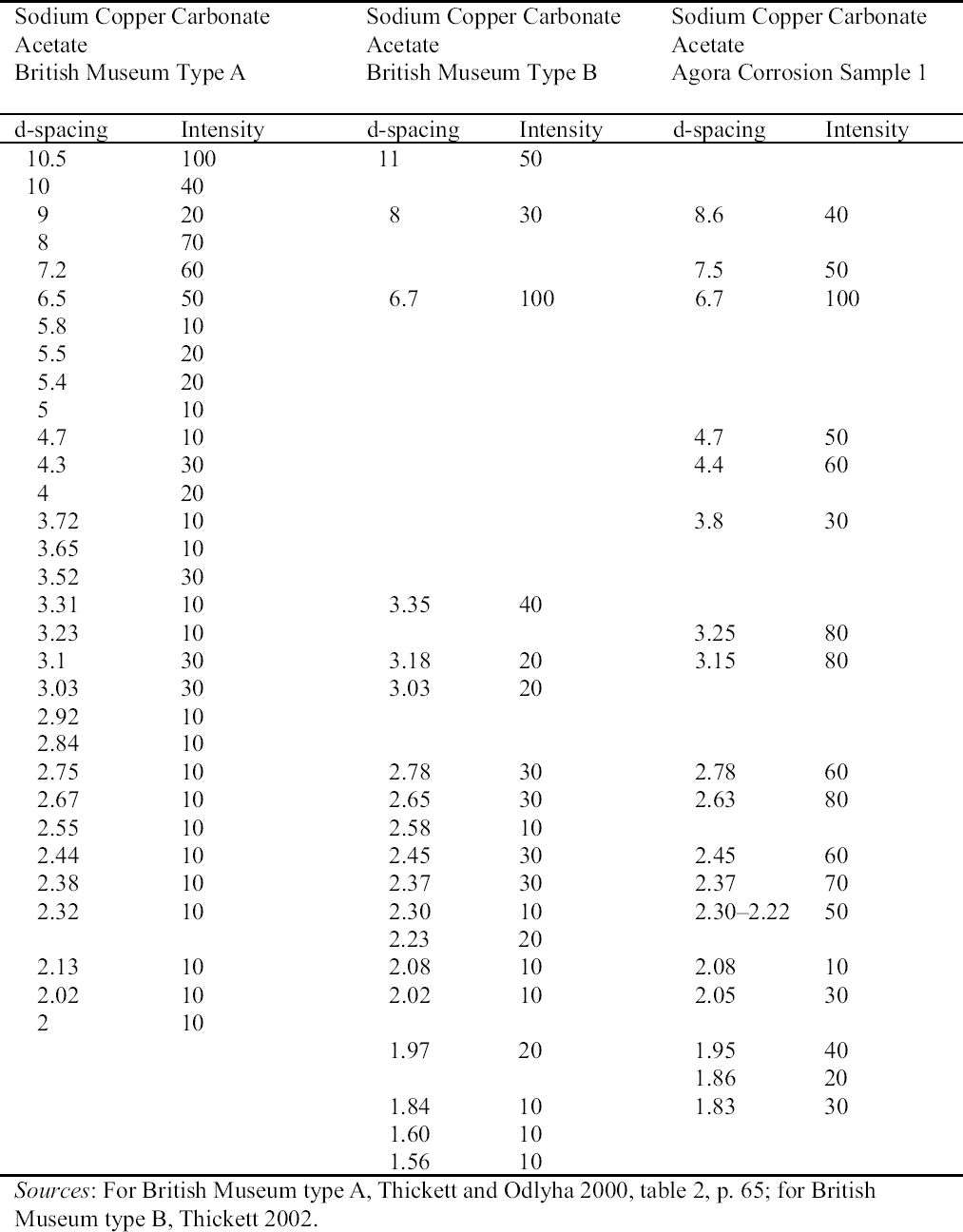
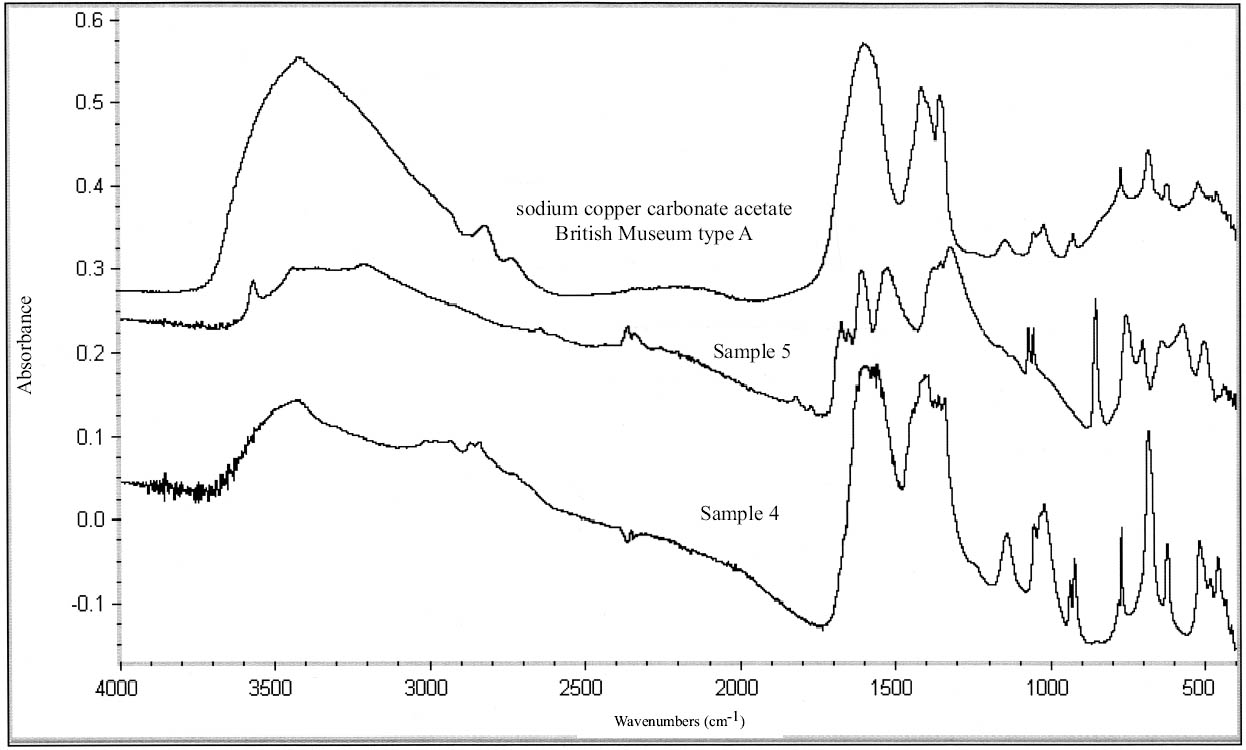
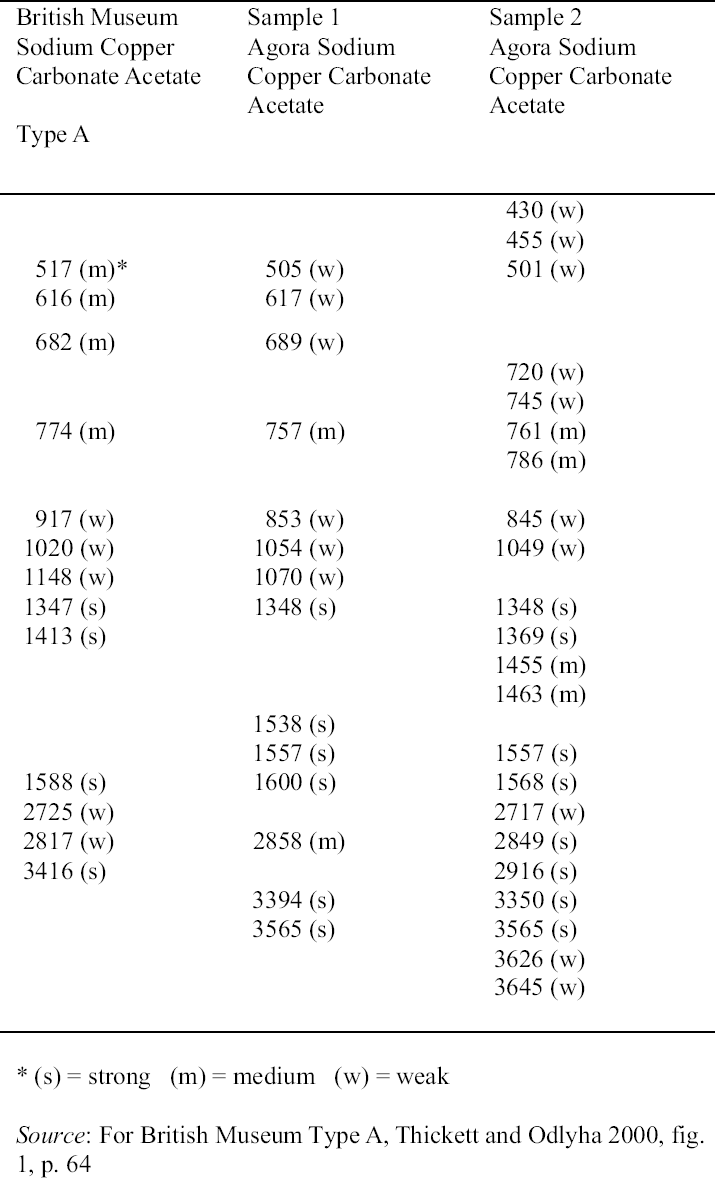
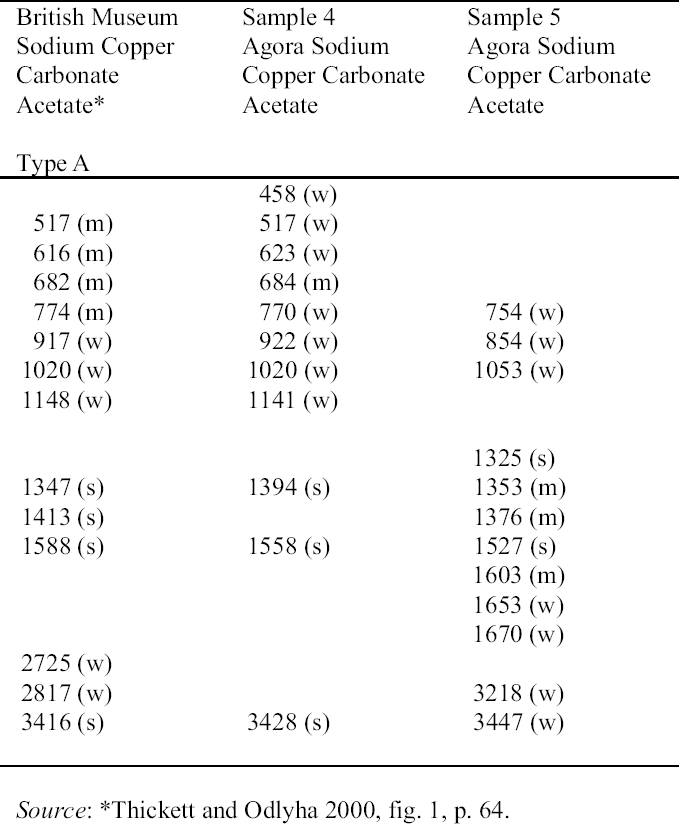
2.3 RESULTS OF CORROSION ANALYSISThe results of the analyses are presented in table 2. The blue corrosion (samples 1, 2, 3, 4, and 5) has been identified as a sodium copper carbonate acetate [NaCu(CO3)(CH3COO)], which presents similarities with the sodium copper acetate compounds found on Egyptian copper alloy objects in the British Museum (Thickett 1998; Thickett et al. 1998; Thickett and Odlyha 2000). An x-ray diffraction pattern for sodium copper carbonate acetate is not available in the International Center for Diffraction (ICD) powder diffraction file. The study of the two sodium copper carbonate acetate compounds found in the British Museum resulted in the publication of type A (Thickett and Odlyha 2000), whereas type B required further characterization. The XRD and FTIR data of types A and B are different and consistent (Thickett 2002). The sodium copper carbonate acetate found in the Agora has been compared to British Museum types A and B. The XRD data for both types of British Museum blue corrosion have been included for comparison with Agora corrosion sample 1 (table 3). FTIR data for the British Museum blue corrosion are compared with Agora corrosion samples 1 and 2 in table 4 and samples 4 and 5 in table 5. The FTIR scan that corresponds to table 4 is shown in figure 4, and the FTIR scan corresponding to table 5 is shown in figure 5. The occurrence of these compounds may become more widely recognized now that the analytical data for their identification have been published. Sample 1 presents similarities with the XRD patterns for both British Museum blue corrosion products, types A and B. Peaks that match type A
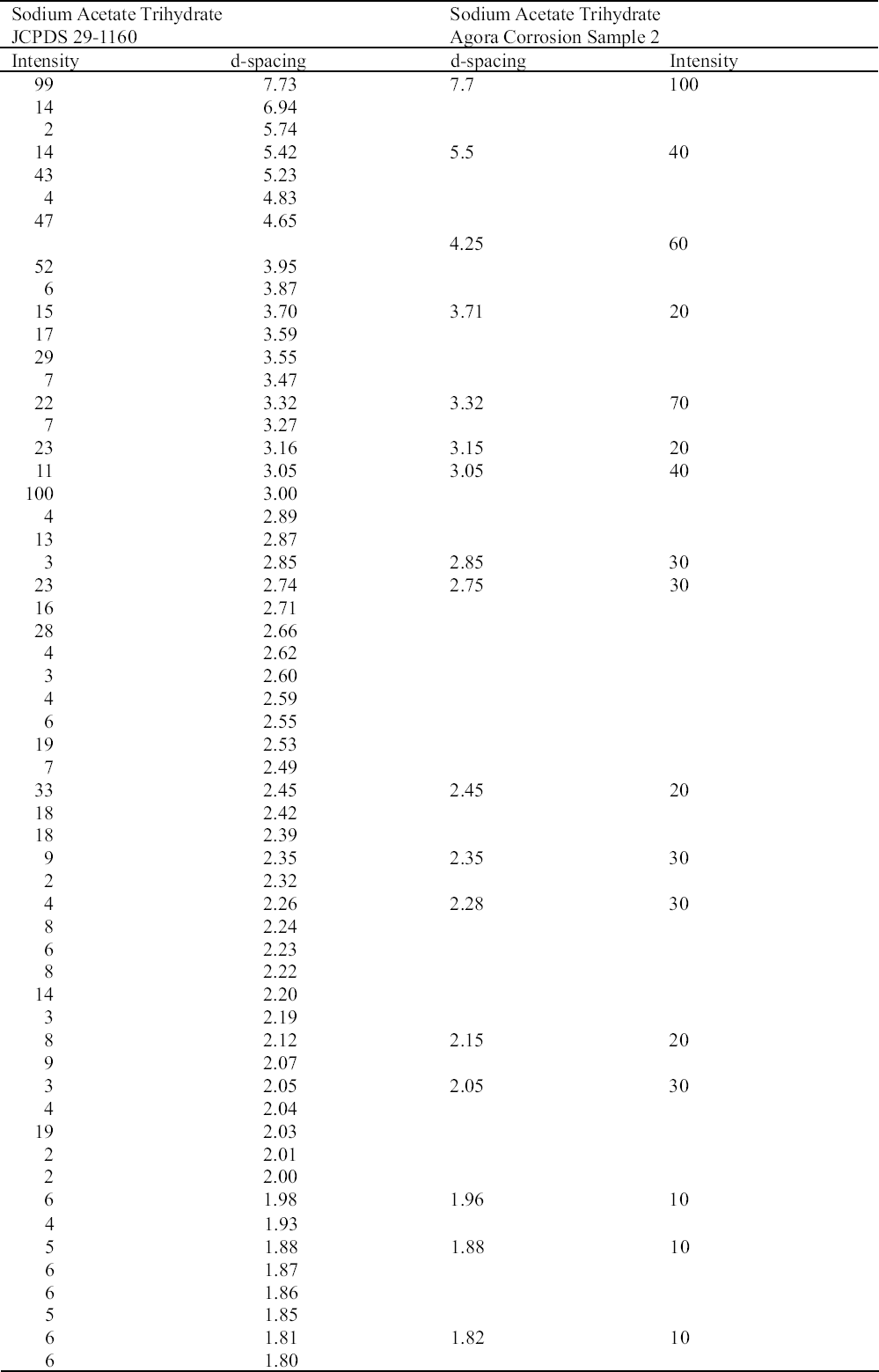
The white corrosion in sample 2 was identified by FTIR as sodium acetate trihydrate (CH3COONa.3H2O) and was confirmed with XRD (table 6). Both FTIR and XRD are not sensitive to components that constitute less than 5–10% of a sample, so such quantities of sodium acetate trihydrate with the sodium copper carbonate acetate would not be detected. Sodium acetate trihydrate has been identified on objects from the Burrell Collection, Glasgow, Scotland, as either needlelike or powdery (Tennent and Baird 1992). The Agora compound presented the same morphologies. In the Burrell Collection this white corrosion was found in association with blue corrosion, as was the case at the Agora and the British Museum (Tennent et al. 1993). The reported solubility of sodium acetate trihydrate in water was confirmed in the Agora (Weast and
3 FACTORS AFFECTING THE DEVELOPMENT OF THESE CORROSION PRODUCTS3.1 SODIUM COPPER CARBONATE ACETATE AND SODIUM ACETATE TRIHYDRATE3.1.1 Influence of Environmental Storage Conditions: Acetic (Ethanoic) AcidSeveral factors have been identified as contributing to the formation of sodium copper carbonate acetate and sodium acetate trihydrate. Sodium may derive from salt in the burial environment, e.g., sodium chloride, or from incomplete removal of conservation materials used in cleaning or stabilization. The copper derives from the copper alloy,
The objects analyzed from the collections of the Agora and the British Museum had been stored in wooden cupboards since the 1930s. The acetic acid concentration in these wooden cupboards in the British Museum and Agora excavations has been measured. A concentration of 1039 � 20 to 1267 � 20 �gm-3 (400 to 500 ppb at 25�C) acetic acid, found in the Agora excavations, is comparable to the levels found at the British Museum: 1071 to 2880 �gm-3(Paterakis 1998; Thickett et al. 1998). Relative
Acetic acid may be adsorbed onto the surface of an artifact and remain inactive, depending on the relative humidity (Donovan and Stringer 1971). Acetate found in the copper (II) hydroxide corrosion of sample 6 by ion chromatography analysis may have resulted from adsorbed acetic acid vapor. Exposure to higher humidity by transfer of the object to another environment or by an increase in RH in the place of contamination may trigger its activity. In this way, corrosion by acetic acid may occur in locations and conditions not characterized by acetic acid contamination (Donovan and Stringer 1971). The concentration of acetic acid may have been much higher when the storage cases were new (Hatchfield 2002). It should be kept in mind that some objects have had up to 70 years in which to develop the acetate corrosion products, although a relatively short time is required for their formation. In the Burrell Collection the sodium acetate trihydrate developed within a three-year period, whereas the associated blue corrosion products developed within a few weeks (Tennent and Baird 1992). Another source of acetic acid could be artificial patination. Chemically stripped objects were sometimes patinated by dipping them in ammonium acetate or by exposing the object to ammonia or acetic acid vapors (Fink and Eldridge 1925). Farnsworth
3.1.2 Influence of Cleaning and Stabilization Materials on the Formation of the Corrosion Products3.1.2.1 SodiumSodium that has contributed to the formation of sodium copper carbonate acetate and sodium acetate trihydrate in the Agora (Paterakis 1999) may derive from (1) the burial environment, (2) chemical cleaning agents such as Calgon (sodium hexametaphosphate/sodium polyphosphate), zinc and sodium hydroxide, alkaline Rochelle salt (5% sodium hydroxide and 15% sodium potassium tartrate), alkaline glycerol (15% sodium hydroxide and 40% glycerin), (3) the stabilization compounds sodium sesquicarbonate and sodium carbonate, or (4) artificial patination with sodium carbonate or bicarbonate (Tennent and Baird 1992) or with sodium sulfite or sodium thiosulfate (Lucas 1932). Specific weight and volume units for the notations of weight (w), volume (v), and % used throughout this text may be found in appendix I, “Strength of Solutions,” in Plenderleith 1956 (343). The discrepancy between the occurrence of sodium copper carbonate acetate and sodium acetate trihydrate on those objects that were not chemically treated in the British Museum and the absence of these compounds on the analogous objects in the Agora may lie in the burial context of the objects. Those artifacts that exhibited acetate corrosion in the British Museum were from Egypt and thus may have been subjected to higher concentrations of sodium salts from the burial environment. There is little documentation of conservation treatments for individual objects in the Agora prior to 1979, rendering the attribution of chemical cleaning and stabilization agents very difficult. We know from general records that a number of cleaning and stabilization compounds containing sodium have been used over the years. Many of these chemical agents were also used on bronzes in the Ashmolean Museum, Oxford, and the Petrie Museum, London, in earlier years (Jaeschke and Jaeschke 1988; Norman 1988). 3.1.2.2 Alkaline Rochelle SaltA few copper alloy objects in the collection were treated, as recorded in the 1950s, with aqueous solutions of alkaline Rochelle salt: sodium hydroxide and Rochelle salt (sodium potassium tartrate). In 1932 and 1956, respectively, Lucas and Plenderleith recommended
3.1.2.3 Sodium Hexametaphosphate/Sodium Polyphosphate (Calgon)Since the 1930s, copper alloy artifacts at the Agora have often been cleaned with Calgon, in concentrations varying from 10% to 35% in water. It is believed that Calgon was originally sodium hexametaphosphate, the composition of which was later changed to sodium polyphosphate. According to the Calgon website, the main active ingredient today is polycarboxylates (www.calgon.com 2002). Six documented treatments with Calgon in the Agora (objects AB177, B1209, ΞΞ 310–ΞΞ 313) have produced sodium copper carbonate acetate. Farnsworth and Plenderleith mentioned the use of 5–15% sodium hexametaphosphate (Calgon) for removing calcareous deposits as well as malachite from copper alloys (Farnsworth 1940; Farnsworth 1949; Plenderleith 1956). Farnsworth attested to the soaking of several small bronze objects in the Agora for a month or more in a cold 5% solution of Calgon (referred to as sodium metaphosphate in 1940) to remove the calcareous deposit. This Calgon treatment is reported not to have altered the patina. She stated that a hot 10% solution could also be used to accelerate the process (Farnsworth 1940). 3.1.2.4 Sodium Carbonate and Sodium
|
 |
Since 1996, Drayman-Weisser's method of inhibiting bronze disease with sodium carbonate followed by the application of benzotriazole (BTA) has been used in the conservation of Agora copper alloys (Drayman-Weisser 1987). It is used as a 5% (w/v) solution in distilled water. Cuprous oxide (yellow-orange) and sodium copper carbonate (chal-conatronite) (blue-green) are two products of the interaction of sodium carbonate with cuprous chloride in this procedure. These are washed away, and the process is repeated until no more copper oxide is produced (Drayman-Weisser 1987). Another product that can sometimes form on the patina is black cupric oxide (tenorite). It probably forms from the oxidation of the cuprous oxide, produced during the conversion of the cuprous chloride (Drayman-Weisser 1987).
Although there are no specifically documented cases for the use of sodium sesquicarbonate in the Agora, it was very likely to have been used, as it is mentioned in some conservation notes (Witherill 1955). In 1932, Lucas recommended prolonged soaking of copper alloys in a solution of 20 parts (w) sodium sesquicarbonate to 100 parts (v) water. Two years later, Plenderleith (1934) recommended it for cleaning and stabilizing copper alloys. He suggested alternating a 5% solution of sodium sesquicarbonate with a 2% solution of citric acid for cleaning and stabilizing, and a 5–25% solution of sodium sesquicarbonate for removing more tenacious encrustation. He also recommended it for local stabilization of bronze disease. Oddy and Hughes (1970) suggested a 5% solution (w/v) in water. It produces the same products as sodium carbonate: chalconatronite, cuprite, and tenorite (Drayman-Weisser 1987; MacLeod 1987; Pollard et al. 1990; Scott 1997). Chalconatronite may have been present in two samples analyzed by XRD in 1998 (Wheeler 1998). As a hydrated compound, it deliquesces when the RH exceeds the equilibrium relative humidity of the compound and crystallizes when the RH falls below this level. It may absorb acetic acid from off-gassing wooden storage cases to form blue sodium copper carbonate acetate (Paterakis 1999). There have been studies relating the use of sodium sesquicarbonate with the formation of sodium acetate trihydrate (Tennent 1992).
3.1.2.5 Sodium Hydroxide in Electrochemical Cleaning and Electrolysis
Electrochemical reduction by zinc and sodium hydroxide was commonly used at the Agora from 1931 on. The Krefting technique, documented by Rathgen in 1905 and Lucas in 1932, was used for cleaning coins by sandwiching them in between sheets of zinc with a sodium hydroxide electrolyte (Rathgen 1905). This technique has been used extensively in the Agora, sometimes with pellets or granules of zinc, for reducing copper alloy objects, including three coins that display the blue corrosion product (N10763 sample 5, N11172, N11308). It appears that many objects cleaned in this way have a dark brown surface on top of which lies the acetate corrosion. One object contains a trace of zinc observed by qualitative EDS that may have resulted from this electrochemical process. Coins have been cleaned as recently as 1982 using this electrochemical method, followed by benzotriazole (BTA) stabilization and Incralac coating; a small percentage have since developed sodium copper carbonate acetate corrosion.
Electrolytic cleaning was carried out with a 5% solution of sodium hydroxide electrolyte, which may also contribute sodium to the corrosion process. There is one instance of blue corrosion on an object (N10746) that underwent this type of cleaning in 1981, followed by treatment with BTA and coating with Incralac.
Another theory for the formation of sodium copper carbonate acetate, distinct from the theory presented in section 3.1.2.4 regarding chalconatronite, is based on the presumed incomplete reduction of the secondary copper corrosion products, such as malachite, during electrochemical or electrolytic cleaning (Bradley and Thickett 1998; Paterakis 1999). Any remaining malachite could serve as a host for the formation of sodium copper carbonate acetate. It is most likely that two factors regarding chemical cleaning are required for the production of blue sodium copper carbonate acetate: the incomplete reduction
3.2 COPPER (II) HYDROXIDE, SPERTINIITE (TURQUOISE BLUE CORROSION PRODUCT)
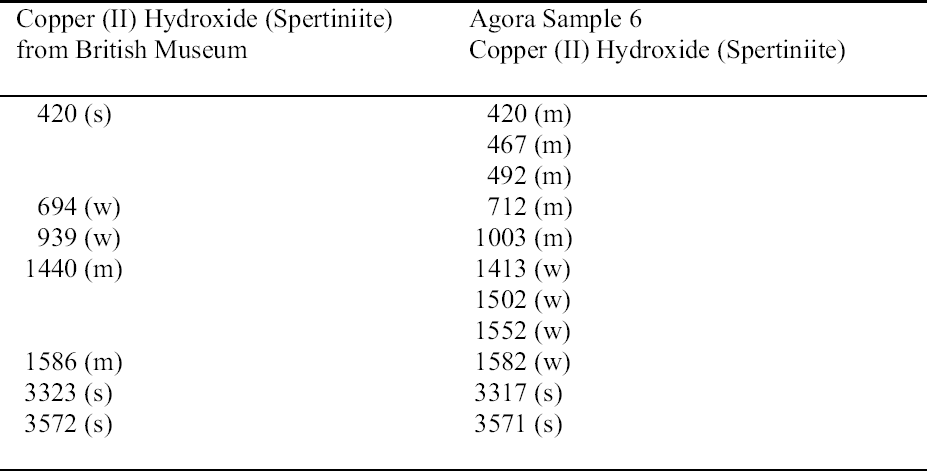
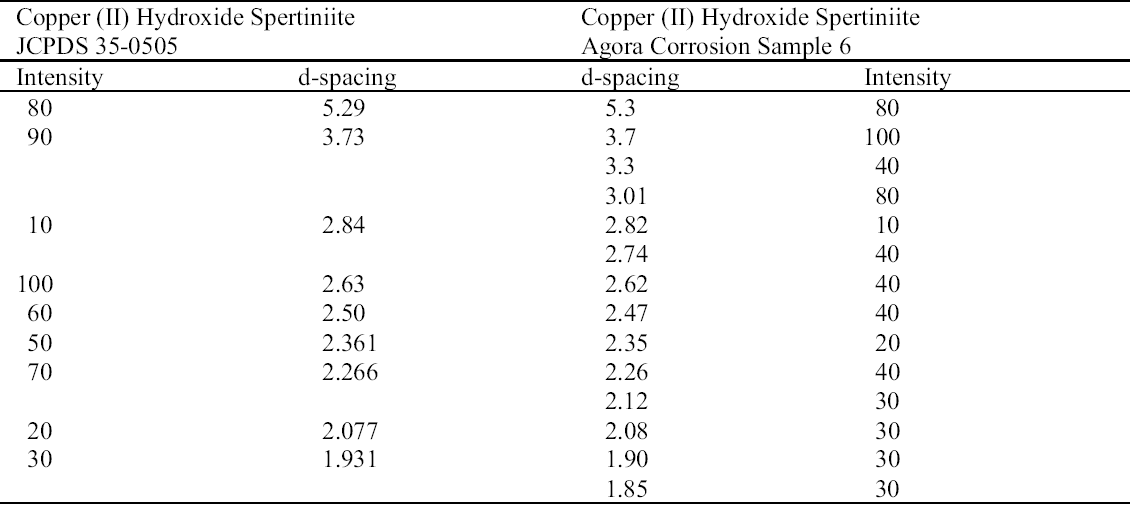
The turquoise blue corrosion product (sample 6) was identified as copper (II) hydroxide, spertiniite (Cu[OH]2) (see fig. 3, page 259). It generally forms on top of the corrosion crust, which is very brittle and readily breaks off, exposing a smooth cuprite surface below. This compound is described as duckegg blue, unstable in the presence of carbon dioxide and water, and poorly crystalline (Scott 1997). In the presence of carbon dioxide it has a tendency to change into copper hydroxy carbonates or, in the presence of chloride, into copper hydroxy chlorides (atacamite, paratacamite) (MacLeod 1999). Only one other instance of copper hydroxide on a copper alloy object has been published, and that was from a marine context (MacLeod 1991). The same compound has been identified by XRD and FTIR on an Asiatic bull in the British Museum (Thickett 1999). If there is a significant amount of moisture present, e.g., in the pores of hygroscopic corrosion products on corroded bronze, the dehydration process will be minimized, thereby stabilizing the copper hydroxide. A high ambient RH may contribute to the moisture present in the corrosion products. In addition to the presence of significant amounts of water, two other factors may support its stability: (1) the local pH and (2) the presence of a mixture of tin (II) and tin (IV) corrosion products on the surface and the presence of degraded metal underneath (MacLeod 1999). MacLeod has found many hydroxy species associated with tin hydrolysis (MacLeod 1999). An additional factor that may stabilize the copper hydroxide is the adsorption of SO2 (MacLeod 1999). The reported insolubility of spertiniite in water was confirmed in the Agora (Weast and Astle 1979).
The use of sodium sesquicarbonate on the object from which sample 6 was taken cannot be ruled out, although sodium was not detected in the analysis. It is possible that copper (II) chlorides and copper hydroxy chlorides, formed from the oxidation of copper (I) chlorides in the corrosion matrix, could be hydrolyzed by sodium sesquicarbonate (depending on the hydroxide concentration in solution), forming copper (II) hydroxide (MacLeod 1999; Paterakis 1999). The fragility of the corrosion crust and the tendency of this crust to separate from a smooth underlying surface may be the result of chemical treatment.
3.3 CASSITERITE AND CUPRITE (DARK BROWN CORROSION)
Cassiterite (SnO2) and cuprite (Cu2O) are the major components of the dark brown corrosion layer (sample 7) (see fig. 2, page 258) that can result from various reactions from cleaning as follows. The primary tin and copper corrosion products present in the original corrosion front would have been exposed by the removal of the secondary corrosion products on the surface during cleaning. The primary oxidation product of tin, Sn2+, may be subjected to hydrolysis and precipitated, or the tin (II) may be further oxidized to tin (IV), which can then undergo hydrolysis. Chemical cleaning of tin and copper corrosion products and the removal of copper (I) chloride complexes (e.g., with sodium sesquicarbonate) result in the hydrolysis of soluble tin (IV) and copper (I) materials that yield cassiterite and cuprite. Treatment with a 10% solution of sodium sesquicar-bonate can cause copper to redeposit as cuprite on the surface of the object, which has been made alkaline by the treatment (Scott 2002). In electrochemical or electrolytic reduction, any nonreduced secondary copper corrosion products, such as malachite, form cuprite (Cu2O) and tenorite (CuO), which appear as a black, powdery product (Scott 1997). The tin corrosion products, which generally are not reduced to tin metal, hydrolyze to produce
While the dark brown and blue corrosion products often occur together, they seem to form independently from one another. The only common factor in their formation appears to be sodium, for example from sodium sesquicarbonate, which could have contributed to the formation of cuprite from copper (I) chloride in the dark brown corrosion (Scott 1997) and of sodium copper carbonate in the blue corrosion.
3.4 THE ROLE OF RELATIVE HUMIDITY AND CONSIDERATIONS FOR THE REMOVAL OF CORROSION PRODUCTS
The relative humidity in the Agora storeroom was monitored over a one-year period and was found to reach a minimum of 40% in the summer and a maximum of 82% in the winter. Daily fluctuations ranged from �2% RH in the summer to 17.5% RH in the winter (Paterakis 1990). The buffering capacity of the wooden cupboards should not be discounted in the consideration of RH inside the cases. The sodium copper carbonate acetate in the British Museum was found to deliquesce at a relative humidity of approximately 65%, indicating its equilibrium relative humidity (eqRH); the eqRH of sodium acetate trihydrate was found to be 75% (Thickett 1998).
As relative humidity has been shown to play an important role in the corrosive activity of volatile acetic acid on metal, considerations in the removal of the acetate corrosion products should take into account the equilibrium relative humidity of the compounds. If the ambient RH is less than the eqRH of the acetate compounds, the corrosion will be in crystalline form, but if the RH meets or exceeds the eqRH, the corrosion crystals will deli-quesce and the salt solution will wick into the pores of the artifact (Thickett 1998; Paterakis 1999). This result is to be avoided. In the Agora the equilibrium relative humidity of the acetate compounds is exceeded in the storeroom in the winter months. Long-term stability of the objects as well as their legibility and aesthetic appearance should be considered in the removal of these corrosion products. Dry (mechanical) removal of the crystalline acetate compounds is preferable to wet removal to prevent spreading in the pores of the object. Alternatively, much of the crystalline sodium copper carbonate acetate can be removed with cotton swabs moistened with ethanol, and the crystalline sodium acetate trihydrate can be removed with water-moistened cotton swabs or poultices. There may be no need to remove the dark brown corrosion, as it often follows the configuration of the surface and is normally not disfiguring. The sodium acetate trihydrate and sodium copper carbonate acetate in the Agora are not necessarily disfiguring but do detract from the legi-bility and aesthetic appearance of the objects. Their removal is recommended if the afflicted objects cannot be stored in a stable relative humidity that is low enough to prevent their deliquescence (i.e., less than the equilibrium relative humidity of these acetate compounds). All metal objects in the Agora are currently being relocated to new powdercoated baked-enamel steel cases in a recently constructed dry storage room. Plastic storage containers maintained with low RH by conditioned silica gel should be used as the primary container for copper alloy artifacts if wooden storage facilities cannot be replaced and if the ambient relative humidity cannot be controlled. The copper (II) hydroxide may be removed mechanically, as it is not water-soluble.
3.5 INFLUENCE OF CONSERVATION COATINGS ON THE FORMATION OF THE CORROSION PRODUCTS
Over the years, many materials in the Agora have been applied to copper alloy artifacts, although there is little documentation predating 1979. A small percentage of the North Slope copper alloys have been coated, however, and a smaller percentage of these display sodium copper carbonate acetate corrosion. Lucas stated that it is best not to use preservative coatings (oils, waxes) on copper alloy objects, but that
Acetate polymer coatings are known to have been used in the Agora, and their effects on the formation of acetate corrosion products have been questioned (Paterakis 1998). Polyvinyl acetates were mentioned by Gettens for use in the conservation of artifacts as early as 1935 (Gettens 1935). According to Gettens, in 1935 the vinyl resins were a rather new material with only a 10-year history of use as film-forming substances. Polyvinyl acetates were also used on copper alloys in the Petrie Museum, London, in the 1950s and 1960s (Jaeschke and Jaeschke 1988).
One coating material in particular, Unichrome Lacquer A140, manufactured by the Metal & Thermit Corporation of New York, was of particular interest, as it is one of the few coating materials recorded in early treatments in the Agora that were identified by manufacturer and product name. An analytical project was carried out to identify the coatings used and to determine the effects of these coatings on the corrosion products, in particular on acetate corrosion.
4 ANALYSIS OF CONSERVATION COATINGS
Examination of the North Slope collection found that the majority of those objects displaying blue corrosion had not been coated. Those objects, characterized by both abundant corrosion and coatings and which were well in the minority, could not be sampled for the coating without removing corrosion as well. In these cases the corrosion masked the coating during analysis, which greatly reduced the number of objects appropriate for sampling. Isolation of the coating by separation from corrosion was not carried out in this project. Information was obtained on the coatings of only two objects on which the blue corrosion was evident under the microscope. As a result of this survey, the question of whether the coatings (as lacquers and consolidants) may have served a protective function on the chemically cleaned objects called for their identification. Conservation coatings on five objects were analyzed by Fourier transform infrared spectroscopy using the same instrumentation and procedures that were employed in the analysis of the corrosion products.
4.1 COATINGS SAMPLED FROM COPPER ALLOY OBJECTS
The objects sampled for coatings and a description of these coatings are presented in table 10. The object from which sample 1 was removed had been chemically or electrochemically cleaned and “soaked in distilled water … then impregnated with A140” (Agora n.d.). The object was virtually free of corrosion except for one spot of blue corrosion. The object from which sample 2 was removed had been chemically or electrochemically cleaned and coated with an unidentified, waxlike material; it was virtually free of corrosion products. The object from which sample 3 was removed was probably not chemically cleaned, as it preserves malachite and cuprite. The coating was crazed and somewhat brittle over much of the object. Those areas that were not crazed adhered well to the surface and were wax-like. The object from which sample 4 was removed had been consolidated with a thick layer of wax. Some fragments, which appeared to have been chemically cleaned and were free of wax, displayed blue on top of dark brown corrosion. The object from which sample 5 was removed had been chemically cleaned and “soaked in distilled water … impregnated with A140” (Agora n.d.). The object was, for the most part, free of corrosion on the surface except for small crystals of sodium copper carbonate acetate in grooves and on the sides of a hole that pierces the object (i.e., those areas protected from contact with a resting surface or handling).
4.2 RESULTS OF COATING
4.2.1 Hydrocarbon Waxes
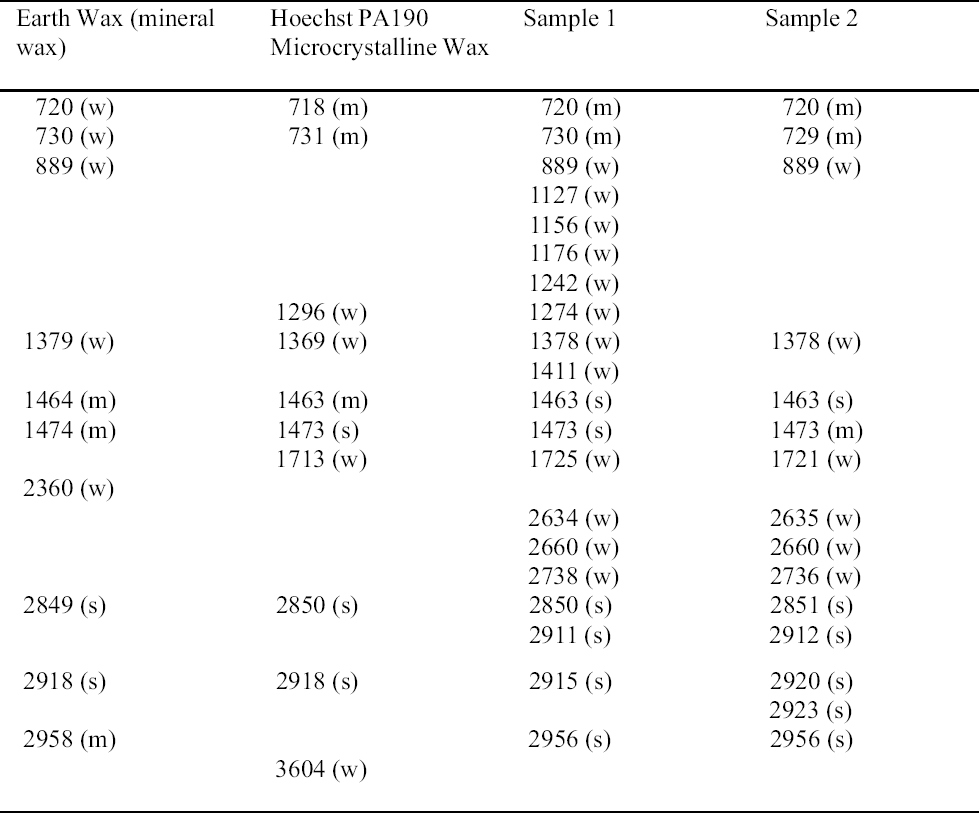
Paraffin wax has been used since the end of the 19th century in conservation, while microcrystalline wax appears in conservation in the 1950s (Plenderleith 1956). Microcrystalline waxes require heating in order to mix with solvents (Horie 1987).
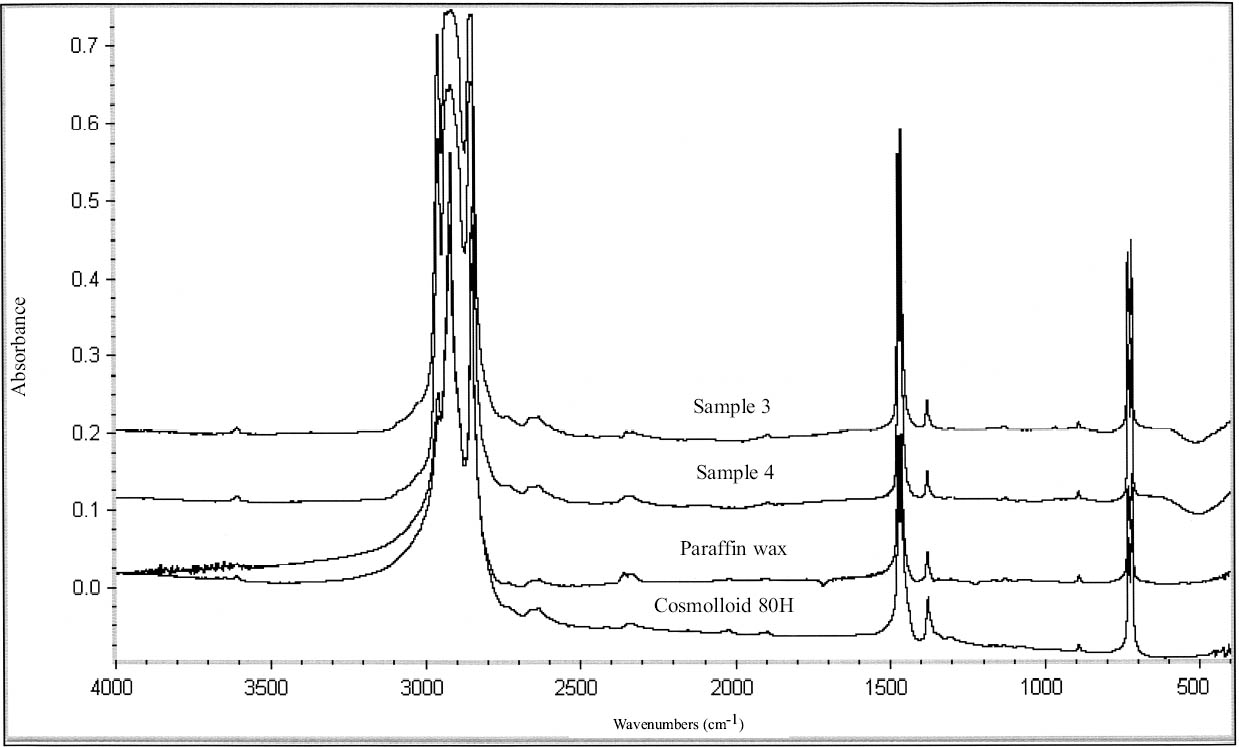
Four of the coating samples (1–4) were identified as hydrocarbon waxes by FTIR analysis (tables 11, 12) (figs. 7, 8). These spectra are characteristic of simple long-chain hydrocarbons with C-H stretching vibrations 2956, 2918, and 2850 cm-1, CH deformations at 1475, 1464, and 1380 cm-1, and absorptions at 720 and 730 cm-1 due to CH rocking (characteristic of long hydrocarbon chains) (Thickett 2002). Samples 1 and 2 show an additional band at 1724 cm-1, probably due to an ester. The spectra of samples 1 and 2 closely resemble the spectra of Earth wax (mineral wax) and Hoechst PA190 (fig. 7) (table 11). Samples 1 and 2 present a peak at wavenumber 1721 and 1725 cm-1, respectively, which distinguishes them from samples 3 and 4. The spectra of samples 1 and 2 match the spectrum of Earth wax at wavenum-bers 720, 730, 889, 1379, 1464, 1474, 2849, 2918, and 2958 cm-1. These samples match the spectrum of Hoechst PA190 wax at wavenumbers 718, 731, 1296, 1369, 1463, 1473, 2850, and 2918 cm-1. Sample 1 was soluble in acetone, chloroform, and 1,1,1-trichloroethane; partially soluble in petroleum benzine; and insoluble in white spirit, toluene, and ethanol. Sample 2 was soluble in chloroform, 1,1,1-trichloroethane, petroleum benzine, and white spirit; partially soluble in toluene; and insoluble in acetone (table 13). Perhaps the discrepancies in solubility may be explained by the higher oxidized state of sample 2 (as revealed by the FTIR scan) or by different components added to the hydrocarbon wax. The two samples were both soluble in chloroform and 1,1,1-trichloroethane.
Samples 3 and 4 were identified as paraffin or microcrystalline wax based on FTIR (see fig. 8). The wavenumbers of these samples matched all those of Hopkins & Williams Filtered paraffin wax and Cosmolloid 80 Hard microcrystalline wax (see table 12). Samples 3 and 4 demonstrated equivalent solu-bility characteristics: both were soluble in 1,1,1-trichloroethane, chloroform, toluene, and petroleum benzine, and insoluble in acetone, ethanol, and white spirit. It is likely that samples 3 and 4 are paraffin wax, as this wax is known to have been used from the earliest days of the Agora excavations (1931). Conservation treatment of these objects may have predated the first use of microcrystalline wax.
It is doubtful that any of the four wax samples (1–4) are pure microcrystalline wax since all four samples were soluble in chloroform and 1,1,1-trichloroethane. Therefore the presence of other
 |
Plenderleith published a wax polish mixture in 1956 that consisted of microcrystalline wax, Cosmolloid 80 Hard (100 g), polyethylene wax, and BASF Wax A (25 g), dissolved in white spirit (Plenderleith 1956). This mixture was prepared by first melting the waxes together and then adding the solvent. By 1971 the mixture was being marketed under the trade name Renaissance Wax (Picreator Enterprises Ltd., London) (Plenderleith and Werner 1971). We may conclude from these results that those individuals treating the objects in the Agora were familiar with the materials used for conservation in the British Museum in the early years, most likely through the publications of Plenderleith and Werner. These publications were also widely used by conservators in America (Drayman-Weisser 1994).
The application of paraffin wax and beeswax (also used in the Agora) to copper alloy objects can cause the formation of organometallic compounds that liberate free fatty acids or dicarboxylic acids. These can combine with copper or lead ions to form metal soaps; copper soaps can promote further corrosion (Paterakis 1996; Scott 2002). Another disadvantage is the irreversibility of wax used for consolidation. As waxes can never be completely removed from a porous object, they interfere with subsequent application of conservation materials. In spite of these drawbacks, it would appear that the hydrocarbon waxes applied to the chemically cleaned metals in the Agora may have protected them from acetic acid attack in storage and the development of acetate corrosion.
4.2.2 CELLULOSE NITRATE
 |
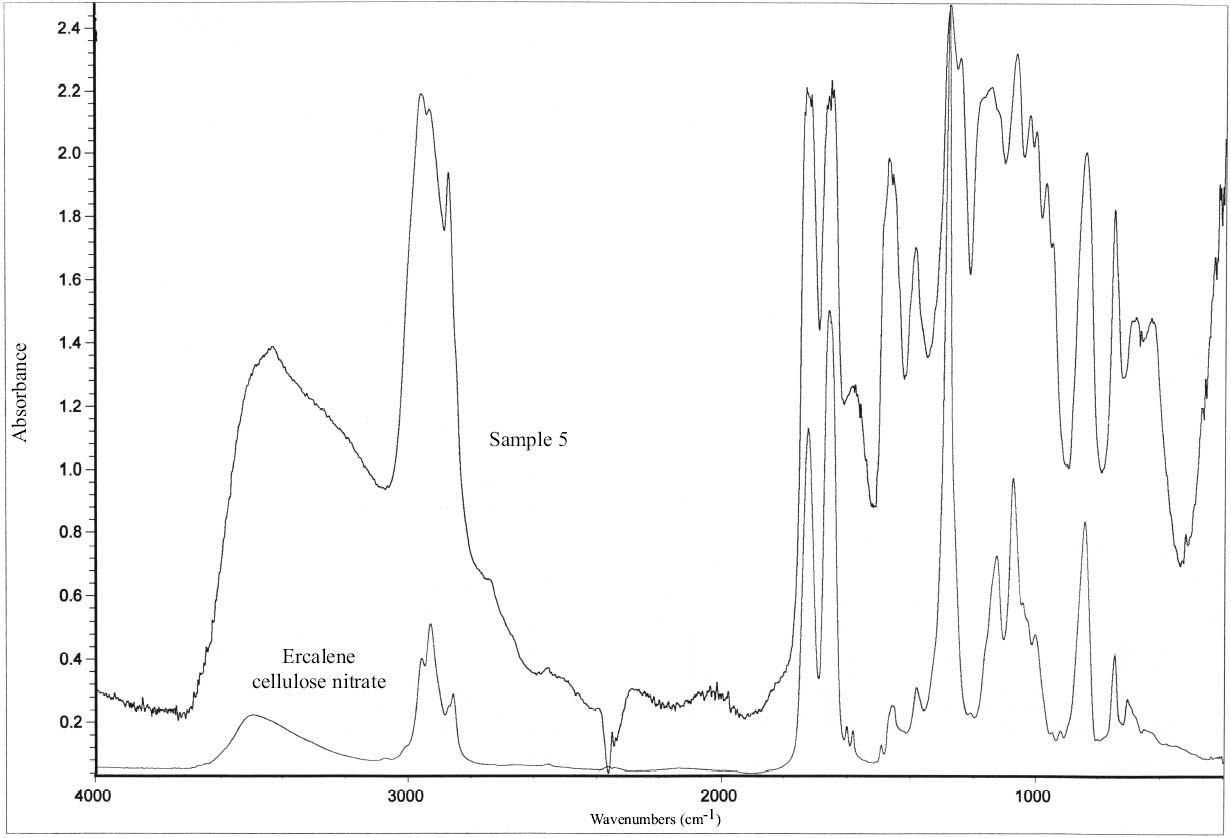
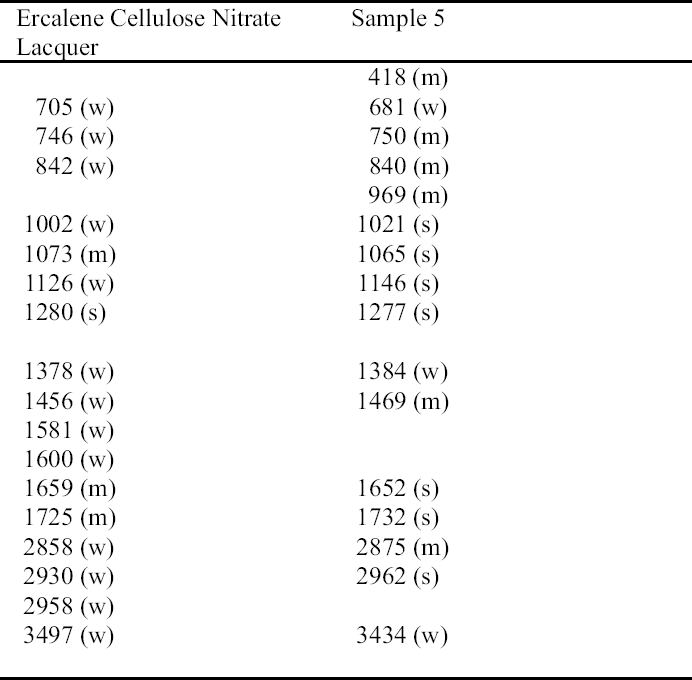
Cellulose nitrate lacquers were made commercially available in 1877 and were first used for conservation in 1899 (Horie 1987; Hatchfield 2002). They have been widely used in the Agora. Cellulose nitrate was identified by FTIR analysis in sample 5 (fig. 9) (table 14). Cellulose nitrate has characteristic asym-metric N=O stretch at 1629 to 1653 cm-1, symmetric stretch at 1285 to 1272 cm-1, N=O stretch from 872 to 841 cm-1, and N=O bending at 761 to 745 and 710 to 689 cm-1 (Thickett 2002). The peak at 1732 cm-1 is a carbonyl stretching due to degradation (Shashoua et al. 1992). Three of the FTIR absorption bands of sample 5—750, 840, and 1277 cm-1—match those of Ercalene, a product that was introduced by Goddards in the 1960s (ICCROM 1963; Scott 2002). Other strong absorption bands
 |
 |
5 OTHER CONSERVATION COATINGS AND SOLUBILITY
Re-examination of conservation materials is particularly valuable for assessing the current state of solu-bility of coatings on copper alloy objects, some of which were applied as early as 1931. Many of the coatings on copper alloys in the Agora collections have remained soluble, and those that have been identifed proved soluble in a range of solvents.
Incralac, containing Paraloid (formerly Acryloid) B-44 and benzotriazole, has been used in the Agora since 1979. In 1982 Incralac was used to lacquer four copper alloy coins (N10763 corrosion sample 5, N11308, N11172, and N10746), which had been electrochemically or electrolytically cleaned in a sodium hydroxide solution, followed by stabilization with benzotriazole. These coins have developed the blue corrosion product described above, and represent the youngest examples of this form of corrosion in the collection. It would seem that the Incralac did not provide a barrier to the acetic acid emissions from the wooden storage cabinets. Thickett noted the
 |
Of the conservation materials used to coat Agora copper alloy objects in the 1950s and 1960s, two were found to have become insoluble. Soluble nylon, marketed under the trade names Maranyl and Calaton, was used to coat copper alloy artifacts at the Agora in the 1950s and 1960s. Since then it has become gummy, attractive to dust, and insoluble. It may be softened in ethanol and then removed mechanically. Shellac, used as an adhesive at the Agora from the 1930s to 1970s, has become insoluble. It may be softened by exposure to acetone: ethanol vapors (1:1) and then removed mechanically.
6 CONCLUSIONS
The characterization of complex copper corrosion compounds containing sodium, copper, carbonate, and acetate is a challenging process that requires extensive analytical work. The exact relationship of a sodium copper carbonate acetate found in the Agora with those in the British Museum has not been clarified by the current study. The determination of the stoichiometric ratio of the components is necessary for a definitive identification.
The identification of corrosion products containing sodium and acetate on copper alloys demonstrates that it is critical to remove all sodium compounds and their residues after cleaning and stabilization treatments and to avoid the use of wood and other organic materials for their storage and display. Hydrocarbon wax coatings may serve a protective function against acetic acid corrosion.
ACKNOWLEDGEMENTS
I wish to thank Kathy Hall for the preliminary examination of the North Slope copper alloys; George Wheeler and Dora Henel for the initial analyses carried out at the Sherman Fairchild Center for Objects Conservation of the Metropolitan Museum of Art, New York; David Thickett for carrying out the analyses and identification of corrosion products and coatings in 1999 at the British Museum, London; Lorraine Gibson for analyzing the acetic acid vapor levels; and Ian MacLeod for his comments on the formation of corrosion products.
 |
 |
 |
REFERENCES
Agora Excavations. N. d. Conservation treatment notes. Agora Excavations, Athens. Unpublished.
Bradley, S., and D.Thickett. 1998. The pollution problem in perspective. In Indoor air pollution: Detection and mitigation of carbonyls, ed. L.Gibson. Amsterdam: Netherlands Institute for Cultural Heritage. 23–26.
Clarke, S. G., and E. E.Longhurst. 1961. The corrosion of metals by acid vapours from wood. Journal of Applied Chemistry11:435–43.
Donovan, P. D., and J.Stringer. 1971. Corrosion of metals and their protection in atmospheres containing organic acid vapours. British Corrosion Journal6(May): 132–38.
Drayman-Weisser, T.1987. The use of sodium carbonate as a pre-treatment for difficult-to-stabilize bronzes. In Recent advances in the conservation and analysis of artifacts, ed. J.Black. London: Summer Schools Press. 105–8.
Drayman-Weisser, T.1994. A perspective on the history of the conservation of archaeological copper alloys in the United States. Journal of the American Institute for Conservation33:141–52.
Farnsworth, M.1940. The use of sodium metaphosphate in cleaning bronzes. Technical Studies in the Field of Fine Arts9:20–24.
Farnsworth, M.1949. The corrosion and cleaning of ancient bronzes. Archaeology (Spring):19–21.
Fink, C. G., and C. H.Eldridge. 1925. The restoration of ancient bronzes and other alloys. New York: Metropolitan Museum of Art.
Gettens, R. J.1935. Polymerized vinyl acetate and related compounds in the restoration of objects of art. Technical Studies in the Field of Fine Arts4:15–27.
Hall, K.1995. North Slope bronzes. Agora Excavations, Athens. Unpublished.
Hatchfield, P.2002. Pollutants in the museum environment. London: Archetype Publications.
Horie, C. V.1987. Materials for conservation. London: Butterworths.
ICCROM. 1963. Synthetic materials used for the conservation of cultural property. Rome: International Center for the Study of the Preservation and Restoration of Cultural Property.
Jaeschke, R., and H.Jaeschke. 1988. Early conservation techniques in the Petrie Museum. In Conservation of ancient Egyptian materials, ed. S. C.Watkins and C. E.Brown. London: Institute of Archaeology Publications, United Kingdom Institute for Conservation Archaeology Section. 17–23.
Lucas, G.1932. Antiques: Their restoration and preservation. 2d ed.rev. London: E. Arnold and Company.
MacLeod, I. D.1987. Conservation of corroded copper alloys: A comparison of new and traditional methods for removing chloride ions. Studies in Conservation32:25–40.
MacLeod, I. D.1991. Identification of corrosion products on non-ferrous metal artifacts recovered from shipwrecks. Studies in Conservation36: 222–34.
MacLeod, I. D.1999. Personal communication. Department of Conservation, Western Australian Museum, Fremantle, Western Australia.
Norman, M.1988. Early conservation techniques and the Ashmolean Museum. In Conservation of ancient Egyptian materials, ed. S. C.Watkins and C. E.Brown. London: United Kingdom Institute for Conservation of Historic and Artistic Works Archaeology Section. 7–16.
Oddy, W. A., and M. J.Hughes. 1970. The stabilization of “active” bronze and iron antiquities by the use of sodium sesquicarbonate. Studies in Conservation
Paterakis, A. B.1990. A preliminary study of salt efflorescence in the collection of the Ancient Agora, Athens, Greece. ICOM Committee for Conservation preprints, 9th Triennial Meeting, Dresden. Los Angeles: ICOM. 2:675–79.
Paterakis, A. B.1996. Conservation: Preservation versus analysis? In Archaeological conservation and its consequences, ed. A.Roy and P.Smith. London: International Institute for the Conservation of Historic and Artistic Works. 143–48.
Paterakis, A. B.1998. Archaeological metals in the ancient Athenian Agora. In Metal 98, ed. W.Mourey and L.Robbiola. London: James and James (Science Publishers) Ltd. 253–59.
Paterakis, A. B.1999. The hidden secrets of copper alloy artifacts in the Athenian Agora. AIC Objects Specialty Group postprints.American Institute for Conservation 27th Annual Meeting, St. Louis. Washington, D. C.: AIC. 70–76.
Plenderleith, H. J.1934. The preservation of antiquities. London: Museums Association.
Plenderleith, H. J.1956. The conservation of antiquities and works of art. London: Oxford University Press.
Plenderleith, H. J., and A. E.Werner. 1971. The conservation of antiquities and works of art. Oxford: Oxford University Press.
Pollard, A. M., R. G.Thomas, and P. A.Williams. 1990. Mineralogical changes arising from the use of aqueous sodium carbonate solutions for the treatment of archaeological copper objects. Studies in Conservation35:148–53.
Rathgen, F.1905. The preservation of antiquities: A handbook for curators. Cambridge: University Press.
Scott, D. A.1997. Copper compounds in metals and colorants: Oxides and hydroxides. Studies in Conservation42:93–100.
Scott, D. A.2002. Copper and bronze in art: Corrosion, colorants, conservation. Los Angeles: Getty Conservation Institute.
Shashoua, Y., S. M.Bradley, and V. D.Daniels. 1992. Degradation of cellulose nitrate adhesive. Studies in Conservation37(2):113–19.
Tennent, N. H.1992. Some applications of ion chromatography to the study of the deterioration of museum artifacts. In Materials issues in art and archaeology, vol. 3. Materials Research Society Symposium Proceedings 267, ed. P. B.Vandiver et al. Pittsburgh: Materials Research Society. 869–82.
Tennent, N. H., and T.Baird. 1992. The identification of acetate efflorescence on bronze antiquities stored in wooden cabinets. Conservator16: 39–43, 47.
Tennent, N. H., B. G.Cooksey, D.Littlejohn, B. J.Ottaway, S. E.Tarling, and M.Vickers. 1993. Unusual corrosion and efflorescence products on bronze and iron antiquities stored in wooden cabinets. In Conservation science in the UK, ed. N. H.Tennent. London: James and James (Science Publishers) Ltd. 60–66.
Thickett, D.1998. Investigation of an unusual pale blue corrosion occurring on Egyptian copper alloy artefacts. Conservation Research Department report no. 1998/18. British Museum, London.
Thickett, D.1999. Personal communication. Conservation Research Department, British Museum, London.
Thickett, D.2002. Personal communication. Conservation Research Department, British Museum, London.
Thickett, D., S.Bradley, and L.Lee. 1998. Assessment of the risks to metal artifacts posed by volatile carbonyl pollutants. In Metal98, ed. W.Mourey and L.Robbiola. London: James and James (Science Publishers) Ltd. 260–64.
Thickett, D., and M.Odlyha. 2000. Note on the
Weast, R. C., and M. J.Astle, eds.1979. CRC handbook of chemistry and physics. 59th edition. Boca Raton: CRC Press.
Wheeler, G.1998. Report on corrosion analysis. Agora Excavations, Athens. Unpublished.
Witherill, L.1955. Conservation notes. Agora Excavations, Athens. Unpublished.
www.calgon.com. 2002. UK, Freqeuently Asked Questions (accessed July 2003).
SOURCES OF MATERIALS
Ercalene, cellulose nitrate lacquer (1963)W. Canning & Co. 77 St. John St. London E.C.1, U.K.
Incralac (1970s), lacquer containing Paraloid (Acry-loid) B-44 and benzotriazoleFrank Joel of London Company no longer exists.
Maranyl or Calaton (1950s), soluble nylonI.C.I. (Imperial Chemical Industries Ltd.) Millbank London S.W.1, U.K.
Unichrome Lacquer A140 (1940s)Metal & Thermit Corporation 100 East 42d St. New York 17, N.Y. Company no longer exists.
Cosmolloid 80 Hard microcrystalline wax (1950s)Astor, Boisellier & Laurence Ltd. 9 Savoy St. London WC2, England
Renaissance Wax (1970s), mixture of Cosmolloid 80 Hard wax and BASF Wax APicreator Enterprises Ltd. 44 Park View Gardens London N.W. 4, U.K.
Earth mineral waxA. F. Suter and Co. Ltd. Thames House 18 Park Street London S.E. 1 9EQ, U.K.
Hoechst PA190 wax (1960s)Hoechst Chemicals Ltd. 50 Jermyn St. London S.W.1, U.K.
Hopkins and Williams Filtered Paraffin waxChadwell Heath Essex, England Company no longer exists.
AUTHOR INFORMATION
ALICE BOCCIA PATERAKIS has an MAC (master of art conservation) from Queen's University in Kingston, Ontario, Canada. Since 1986 she has been head of conservation of the Agora Excavations for the American School of Classical Studies in Athens, Greece. She has carried out research into the contamination of ceramics by soluble salts and the corrosion of copper alloys, with particular interest in determining the criteria for the formation and behavior of these compounds. Address: Agora Excavations, American School of Classical Studies, 54 Souidias St., Athens 10676 Greece.
 Section Index
Section Index