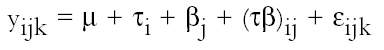ANTIFUNGAL PROTECTION AND SIZING OF PAPER WITH CHITOSAN SALTS AND CELLULOSE ETHERS. PART 2, ANTIFUNGAL EFFECTSMARIA DEL PILAR PONCE-JIM�NEZ, FERNANDO A. L�PEZ-DELLAMARY TORAL, & HUMBERTO GUTIERREZ-PULIDO
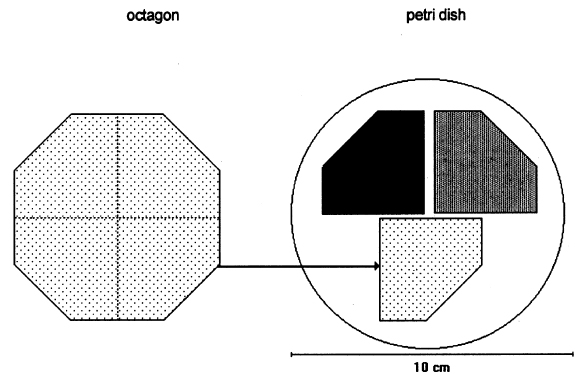
2 METHODOLOGYThe methodology we used for the antifungal tests was inspired by TAPPI standard T487 om-93 (TAPPI 1993a), which is a method for determining antifungal resistance of paper and paperboard, mainly when they have been chemically treated against fungi. The standard describes two methods: the “spore-mycelia” TAPPI standard T487 om-93 (TAPPI 1993a) recommends using three strains of fungi of the species: Chaetomium globosum, Aspergillus niger, and Aspergillus terreus. Unfortunately, the specific strains are very difficult to obtain, but the same species of test organisms were employed. 2.1 SAMPLES OF PAPERWhatman no. 1 filter paper was used because it is a pure cellulose paper, free of additives, and a point of reference in respect to other experimental works. The choice of paper samples to size with polymers was done in accordance with TAPPI standard T400 om-90 (TAPPI 1990). The shape of the filter paper used for the samples was different from that indicated in the standard. For example, 420 mm2 octagons were used in place of 50 mm2 squares (see appendix for other modifications). After the polymer sizing dried, each octagon was separated into four sections, forming pentagons of 105 mm2. Three samples were placed in each Petri dish (fig. 1). Each sample was numbered with a graphite pencil according to the numbers in table 1 that indicate the kind of sizing and the fungal strain assigned. Each sample was also marked with lines in the cross direction of the paper, with 1.5 cm separation between lines. For the antifungal test, one-fourth of an octagon was used for each sample; for the zero-span tensile strength, strips 1.5 cm wide were used. Testing was done along the cross direction of the paper.
After sizing, the samples were disinfected with ethylene oxide (EtO) as described below and were placed in Petri dishes as shown in figure 1. Each section of paper had a different consolidant. The samples were injected with a 2% solution of each of the polymers indicated in section 2.2. After the samples were sized with a roll hand-coater no. 8 (for printing ink tests), they were left to dry over a nylon screen and then neutralized using ammonia vapors in a closed chamber for 20 minutes. Sterilization of the paper samples was done in a hospital clinic using ethylene oxide. This was the method that least affected properties of the paper (autoclave and ozone were also evaluated for sterilization, but autoclave decreases the resistance and brightness of paper, and ozone is not effective). 2.2 CONSOLIDANT SIZESThe chitosan was prepared from deproteinized and deacidified shrimp shells by treatment with alkali in two steps to diminish its content of N-acetyl groups (percentage of free primary amines). The acetylation degree of the sample was 17.3%; the viscosity (a capillary viscometer) of a 1% (w/v) in 1% (v/v) acetic acid solution was 51 cps; and its content in ashes was 0.23% (L�pez-Dellamary 1996). Three different 2% (w/v) solutions of chitosan were used, employing in each case glacial acetic acid, butyric acid, and propionic acid to dissolve the chitosan. Thus we prepared three salts: chitosan acetate (AQ), chitosan butyrate (BQ), and chitosan propionate (PQ). The preparation consisted of adding 2 g of shredded chitosan to 100 ml of deionized water, then stirring the mixture while adding drop by drop 1 ml of the corresponding acid to completely dissolve the chitosan. Preparation of 2% solutions of chitosan salts and cellulose ethers is described in part 1 of this article (Ponce-Jim�nez et al. 2002).
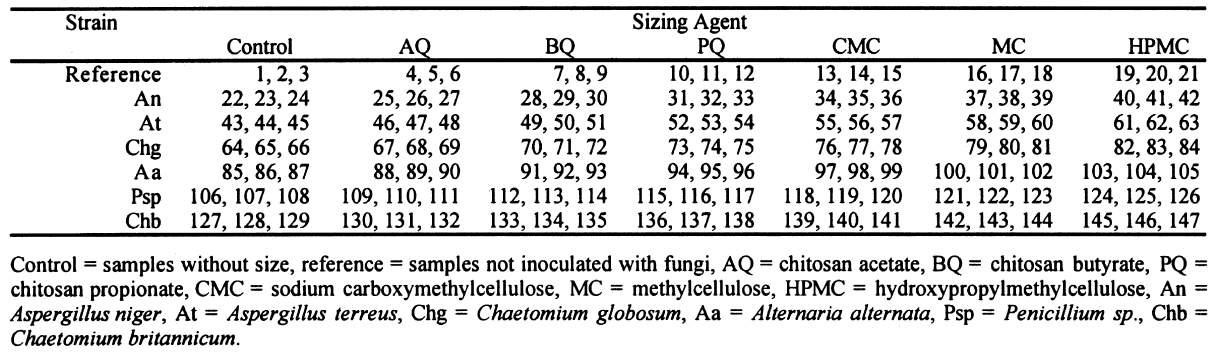
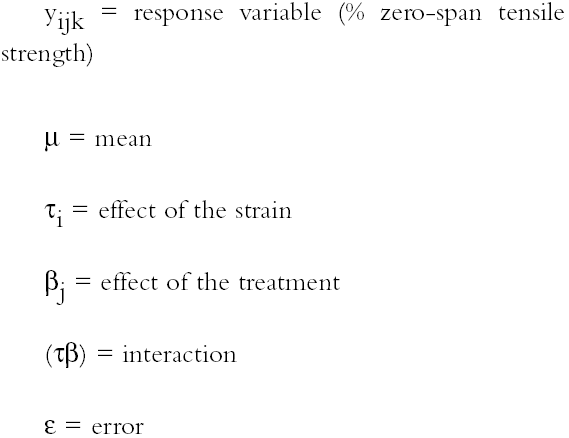
2.3 FUNGAL STRAINSIn the preliminary stages of this work, we did cellulolytic activity tests on 16 strains that were isolated from 17th–20th-century documents from the Archive Historical of Notaries, Mexico City, that showed deterioration by fungi. Two different techniques were used to make our determination: loss of mass and degree of polymerization (see appendix). Both tests were done on pulp samples of Whatman no. 1 filter paper with the 16 isolated strains. The results of both tests nearly coincide in the cellulolytic activity of each strain. Three of the most cellulolytic strains were selected for the biotests and identified in the Mycology Laboratory of Polytechnic National Institute, Mexico City, as Alternaria alternata, Penicillium sp., and Chaetomium britannicum. Methods of determining percentage of mass loss and degree of polymerization are described in the appendix. In the tests for antifungal protection, the following strains of fungi were utilized to induce deterioration on the paper: Chaetomium globosum (Chg), Aspergillus niger (An), Aspergillus terreus (At), Alternaria alternata (Aa), Penicillium sp. (Psp), and Chaetomium britannicum (Chb). Reference samples for the tests were not inoculated with fungi. The control samples in the group of treatments or coatings were without any type of size consolidant. All strains were grown on potato-dextrose-agar medium at 28�C and 100% RH in Petri dishes and culture tubes. The strains were grown in test tubes for 14 days, after which suspensions of the spores and mycelium were prepared and inoculated on the paper samples in accordance with TAPPI standard T487 om-93 (TAPPI 1993a), with some slight variations (see appendix). To determine the antifungal resistance, the EtO-sterilized polymer-coated paper samples were placed on agar mineral salts, and 15 ml of culture medium was placed in each Petri dish. Later, the samples of paper were put on the solid medium in a sterile field, and they were kept in hermetic boxes at 28�C. After 24 hours, 1 ml of each spore-mycelia suspension was inoculated with sterile disposable syringes in the sterile field inside a laminar flow chamber (hood). A new syringe was used to inoculate each strain. During incubation, the samples were placed in hermetically closed plastic boxes. The Petri dishes were arrayed in reverse position on a raised screen to form a space that allowed a volume of water to be retained in the bottom of the box, maintaining an RH near 100%, with a temperature of 28�C, and in darkness. The samples were kept completely moist for the total incubation period of 21 days. 2.4 TREATMENT OF THE SAMPLES WITH 70% ETHYL ALCOHOL (ASEPTIZATION)To be able to handle the samples aseptically after 2.5 ZERO-SPAN TENSILE STRENGTH AS A TEST OF DETERIORATIONThe zero-span tests were done with a modified pendulum-type tensile strength machine, and strips 1.5 cm wide were utilized in accord with TAPPI T231 cm-85 (TAPPI 1985). Zero-span tensile strength is different from folding endurance in that it is related more to the intrinsic resistance of the fibers than to the cohesive force between them. So measuring the force necessary to break the paper shows the resistance of the fiber, not the resistance of the fiber-to-fiber bonding. The protection given by the consolidants was evaluated by zero-span tensile strength because the intrinsic resistance of the fibers is in intimate relation with its state of conservation. Submitting the paper to enzymatic or aging deterioration will affect the zero-span tensile strength. 2.6 FACTORIAL EXPERIMENTAL DESIGN 7 X 7To determine the effect caused by each one of the strains and their combined effect with each one of the coatings, we did a factorial experimental design of 7 � 7 factors, obtaining 49 different combinations. In table 1, the column heads list the kinds of coating, and the row headings list the types of fungi strain. In table 2 the order of assignment of each Petri dish is indicated. This order was obtained by randomizing the total number of samples (three samples were placed in each dish). The mathematical model for this experimental design is:
Each test was repeated three times. The total number of samples was calculated as follows: 7 � 7 = 49 (3 repetitions) = 147 samples. This design is applied to each response variable, in this case for the percentage of zero-span tensile strength. At the end of the experimental treatment, the readings of zero-span tensile strength were put into a computer and processed using Statgraphics version 7, filling three columns with data: (1) type of strain, (2) consolidant size, and (3) zero-span tensile strength. Analysis of variance (ANOVA, for more than one variable) was done for strain, size, and their interactions (table 3). Figures 2 and 3 compare the means. The interaction effect of size and strain on zero-span tensile strength is shown in figure 4. |