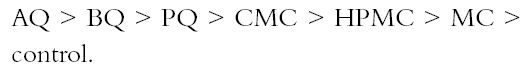ANTIFUNGAL PROTECTION AND SIZING OF PAPER WITH CHITOSAN SALTS AND CELLULOSE ETHERS. PART 1, PHYSICAL EFFECTSMARIA DEL PILAR PONCE-JIM�NEZ, FERNANDO A. L�PEZ-DELLAMARY TORAL, & EZEQUIEL DELGADO FORNU�
5 DISCUSSIONWhere the polymers used for coatings are concerned, the difference in viscosity between cellulose ethers and chitosan salts is evident (see table 1). This difference determines the efficacy of impregnation of the paper's fiber mat. A total impregnation can form a homogeneous and complete layer, which constitutes a good protective mechanism against micro-organisms (Guilleumas 1972). Further, the lower viscosity of chitosan salts provides better coatings than cellulose ethers. However, the results depend on the degree of polymerization, the degree of substitution (deacetylation in the case of chitosan) of the polymers involved, and the solution concentration. Another property taken into consideration was the pH of the 2% solutions. The chitosan salt solutions have a pronounced acidity because acids are employed in their formation. This acidity was inevitable because the maximum pH to solubilize chitosan was 5.7–5.8. These solutions precipitate easily at the slightest increase of pH. Neutralization probably will reinforce formation of a film on the paper's fibrous net. Allan et al. performed tests by depositioning chitosan in pulp slurries at pH 5. The slurries were then brought to pH 10 to precipitate the polymer on the fibers. But a “loss in brightness is associated with the increase in pH required to precipitate the chitosan” (Allan et al. 1977, 783). Nevertheless, it will be necessary to research the effects of a higher pH on the chitosan's ability to retard fungal growth and its long-term physical effects on the properties of the paper. To maintain paper stability, the preferred condition for preparing cellulose ethers is a neutral pH. However, the low pH of the chitosan-treated papers may be conveniently neutralized later, or a modified chitosan that is soluble in neutral aqueous solution could be used. Both of these approaches are being researched for their effects on the retardation of fungal growth and on the physical properties of the paper support, and the results will be published. Both chitosan salts and cellulose ethers increased the strength properties of Whatman no. 1 filter paper (see fig. 2). The folding endurance depends on the flexibility of the paper sheet and the film formed by the polymer applied. Both cellulose ethers and chitosan salts increase folding endurance, but chitosan to a lesser extent. This disparity is possibly due to the shorter polymer chain length of the chitosan used, as the solution's lower viscosity suggests (see table 1 and fig. 3).
Figure 3 shows that the cellulose ether coatings are superior to the chitosan in the folding test. Nevertheless, the standard deviation indicates that the coatings with cellulose ethers are more heterogeneous. Still, the chitosan has lower standard deviation even though the same method of application was Page (1969) devised a useful theory to calculate the relative contributions of the individual fiber resistance and of fiber bonding for tensile resistance. Page's theory requires measuring zero-span tensile strength, which reflects the intrinsic resistance of the paper. The standard deviations in figure 2 are lower compared with those in figure 3. The result of the test of zero-span tensile strength is the best indicator of the paper's state of conservation and is a more direct measurement of the fiber resistance. Table 2 presents the brightness averages and standard deviations before and after sterilization. Chitosan salts notably diminished the brightness of the paper, but cellulose ethers did not; the brightness value stayed within an acceptable range, with an almost invisible change. However, after sterilization with EtO, the whiteness decreased in all cases, including the control sample, and the diminishing was proportionate to the brightness value before sterilization. The only coating that maintained its brightness above 90% was MC. The chitosan used in this research was not bleached, and it likely contained impurities. Under conditions of high humidity and 28�C, a light (reddish) discoloration appeared in samples. It is unlikely that this result will impede the use of chitosan in conservation works, given that clean, high-quality chitosan with excellent transparency can be obtained (Muzzarelli et al. 1981). When the other tests were performed, it was found that the sterilization with EtO did not substantially affect the results. Zero-span tensile strength was practically unchanged, although some samples underwent overall strengthening, e.g., those coated with HPMC and CMC and the controls. There could be a cross-linkage between the polymeric chains of the cellulose and those of the cellulose ethers, a possibility that should be investigated further. In the folding endurance test, sterilization with EtO slightly reduced the elasticity of the fibers in papers coated with cellulose ethers. In the chitosancoated papers, the effect on the elasticity was less evident, particularly in the case of PQ (see fig. 3). On the other hand, the coatings' negligible effect on zero-span tensile strength reflects the resistance of the individual fibers. Table 2 shows that the sterilization diminished the brightness of the paper in one or two points. The samples with the greatest loss of whiteness after sterilization were those treated with CMC, HPMC, and MC. The second greatest loss was on the control and BQ, AQ, and PQ. This result conflicts with results before sterilization, in which the coatings diminished the brightness of the paper in the following order:
Before sterilization, the chitosan-treated papers had a greater degree of diminished brightness than the cellulose ethers had, but after sterilization with EtO, papers with cellulose ethers had a greater loss of brightness than with the chitosan salts. The results in table 3 show a decrease of pH after sterilization with EtO, which is similar in all samples except BQ and PQ. Acidity is deleterious to paper because it promotes acid hydrolysis of cellulose chains and is one of the principal mechanisms of aging and deterioration of resistance properties. This effect implies that if chitosan is used in the strengthening of paper, a deacidification of the paper would be necessary either by adding ammonia vapors in a closed chamber or finding another method that would produce a neutral pH. This need for deacidification could be decreased by making new derivatives of chitosan with neutral or slightly alkaline pH levels, which would conserve all properties with favorable characteristics. |